|
|
|
 ROCKVILLE, MARYLAND, June 4, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, is proud to announce the publication of the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide was developed by BHI, the Economic Alliance of Greater Baltimore and the Montgomery County Department of Economic Development, in collaboration with the Baltimore Business Journal. ROCKVILLE, MARYLAND, June 4, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, is proud to announce the publication of the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide was developed by BHI, the Economic Alliance of Greater Baltimore and the Montgomery County Department of Economic Development, in collaboration with the Baltimore Business Journal.
The Guide serves as a compendium of resources to biohealth innovators and entrepreneurs working to start and grow new companies and technologies in the region. This essential entrepreneurial resource includes a compilation of information on financial resources, university facilities and programs, economic development programs, and existing federal laboratory facilities and programs, as well as how to work with these assets. The Guide is one of the many projects BHI is developing to successfully transform the regional environment for biohealth startups through harnessing Central Maryland’s biohealth assets and establishing an entrepreneurial ecosystem.
The Guide has been distributed through the Baltimore and Washington business journal subscribers, and is being distributed to all regional partners. For your copy of the guide please download here or contact BioHealth Innovation for details on how to receive a hard copy.
back to top 

BioHealth Innovation is working to assist relevant SBIR projects through its Commercial Relevance Program for the August 5th, 2013 NIH SBIR Deadline.
If your company is planning a submission for the August 5 deadline, BioHealth Innovation can assist with your submission. Through the BHI Commercial Relevance Program for SBIR/STTR projects select companies submit their federal funding concepts and receive pre-proposal feedback to help strengthen your application. Further support from BHI’s network of professional consultants and service providers is available to assist in improving your application. If you’re are planning a submission for the August 5 deadline contact Ethan Byler for details on possible support from BHI.
NIH SBIR Phase I Program
- Companies to complete brief application describing the proposed SBIR project apply by June 18th
- All companies notified of SBIR assistance by June 26th
- Guidance, writing strategy, direction, and review sessions to be schedule to enable you through grant submission
- Feedback and redlined comments on written drafts and proposals materials as well as final submission assistance
back to top 

BioHeaIth Innovation aims to transform Central Maryland into a commercialization hub by building an entrepreneurial ecosystem using the research, funding, networks and business development resources available in the region.
The market for Health Information Technology concepts and new products are moving at a rapid pace. Since the signing of the Health Information Technology for Economic and Clinical Health Act (HITECH) by President Obama in 2009 that allocated $36 billion to aid investments in healthcare IT and spurring new initiatives like the Office of the National Coordinator for Health Information Technology (ONC) the environment and funding available to test new products significantly escalated.
Fast forward four years—there remains to be enormous untapped market opportunity with many new products being brought to fruition and highly innovative new concepts under development by the private and public sectors.
Maryland, a significant center of the health care industry nationally and home to the National Institutes of Health, Food and Drug Administration, Johns Hopkins University, the University System of Maryland, and a robust private sector has emerging opportunities within the Health IT industry sector from telemedicine and remote monitoring to electronic medical records and mobile health.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
Request for Applications (RFAs):
- RFA-TW-13-002: Research on the Role of Epigenetics in Social, Behavioral, Environmental and Biological Relationships, throughout the Life-Span and across Generations (R21)
This Funding Opportunity Announcement (FOA) encourages exploratory and developmental grant applications to lay the foundation for innovative and collaborative basic research on the role of epigenetics in social, behavioral, environmental and biological relationships, throughout the life-span and across generations. Research plans that are responsive to this FOA will use existing bio-psycho-social and environmental data from human cohorts or animal studies that have biospecimens available for epigenetic profiling.
- RFA-HL-14-015: Clinical Coordinating Center (CCC) for the NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network (U01)
This funding opportunity announcement (FOA) is for a Clinical Coordinating Center (CCC) for a new National Heart, Lung, and Blood Institute (NHLBI) Clinical Trials Network for the Prevention and Early Treatment of Acute Lung Injury (ALI) (PETAL Network). The Network will develop and conduct randomized controlled clinical trials to prevent, treat, and/or improve the outcome of adult patients with, or at risk, for ALI or the Acute Respiratory Distress Syndrome (ARDS). This FOA solicits applications for a CCC and runs parallel with a separate FOA for the CCs (RFA-HL-14-014).
- RFA-HL-14-014: Clinical Centers (CC) for the NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network (U01)
This funding opportunity announcement (FOA) is for Clinical Centers (CC) for a new National Heart, Lung, and Blood Institute (NHLBI) Clinical Trials Network for the Prevention and Early Treatment of Acute Lung Injury (ALI) (PETAL Network). The Network will develop and conduct at least 3-5 randomized controlled clinical trials to prevent, treat, and/or improve the outcome of adult patients with, or at risk for, ALI or the acute respiratory distress syndrome (ARDS). This FOA solicits applications for CCs and runs parallel with a separate FOA for the CCC (RFA-HL-14-015).
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 
The U.S. Department of Health and Human Services, Biomedical Advanced Research and Development Authority (BARDA) has entered into a  collaborative agreement with Glaxo Smith Kline (GSK) to evaluate the efficacy and safety of the company’s portfolio of clinical stage antibacterial assets for treating hospital and biothreat infections. collaborative agreement with Glaxo Smith Kline (GSK) to evaluate the efficacy and safety of the company’s portfolio of clinical stage antibacterial assets for treating hospital and biothreat infections.
The contract is unique in that it is the first in which BARDA has taken a portfolio approach to funding drug development with industry.
back to top 
 Through new agreements with the Canadian BC Cancer Agency and Columbia University, the company now has access to biomarkers for lymphoma, glioblastoma, and several other cancers. These biomarkers will be developed into diagnostic assays that can assist physicians in choosing targeted treatments, Qiagen says. Through new agreements with the Canadian BC Cancer Agency and Columbia University, the company now has access to biomarkers for lymphoma, glioblastoma, and several other cancers. These biomarkers will be developed into diagnostic assays that can assist physicians in choosing targeted treatments, Qiagen says.
The company’s glioblastoma cancer biomarker, acquired from Columbia University, detects the presence of FGFR-TACC fusion genes. The lymphoma biomarker, acquired from BC Cancer Agency, detects the Y641 EZH2 gene mutation. This type of lymphoma is targeted by pharmaceutical manufacturers like Epizyme and Constellation.
back to top 
By Eve Green
Partner companies Theravance and the GlaxoSmithKline have received Food and Drug Administration clearance for their new drug for the treatment of COPD, Breo Ellipta. COPD encompasses what used to be known as emphysema and chronic bronchitis – the new drug should be an improvement on current treatment options which can ease the symptoms of this disease. COPD, which is strongly linked to smoking, is the third most significant cause of death in America.
Curtis Rosebraugh, director of FDA Office of Drug Evaluation II, commented that this new long-term maintenance treatment for COPD will provide new care options for the millions of people in the USA who suffer from the condition. The companies have stated that the drug should be available during the third quarter of 2013. Breo Ellipta, which requires a single dose per day, should surpass existing treatments which require two doses.
COPD
COPD stands for chronic obstructive pulmonary disease, a progressive condition (one which continually worsens) which causes breathing problems in those who suffer from it. The symptoms are coughing and the associated overproduction of mucus, shortness of breath especially during exercise and tightness of chest. COPD is also associated with a greater susceptibility to chest-infections. Doctors used to refer to ’emphysema’ and ‘chronic bronchitis’, though these are now officially grouped under this general condition.
A disease which is currently under-diagnosed, this condition refers to the inflammation and subsequent damage to the interior of the lungs, and results in lower efficiency in taking on oxygen and in problems with the mechanics of breathing. Smoking is the top cause of COPD, though a genetic predisposition can cause it in some people. While the damage which it causes cannot be repaired, sufferers who subsequently quit smoking can slow down the progress of the disease.
back to top 

QIAGEN N.V. (NASDAQ: QGEN; Frankfurt Prime Standard: QIA) today announced two agreements adding promising new biomarkers involving glioblastoma, lymphoma and other cancers to QIAGEN’s expanding portfolio of potential companion diagnostics that is being developed to help doctors use a patient’s genomic information to guide treatment decisions.
In the glioblastoma project, QIAGEN has entered into an exclusive worldwide licensing option on FGFR-TACC fusion genes with Columbia University in New York. QIAGEN intends to develop this biomarker into a diagnostic test for routine use in diagnostic workups, which may enable doctors to identify glioblastoma patients who could benefit from targeted treatments now under development. Glioblastoma is the most common and aggressive form of primary brain tumor, a serious unmet medical need because the disease is generally fatal despite aggressive therapy. Fusions between members of the FGFR and TACC gene families also have been identified recently as present in several other malignancies, including bladder cancers.
back to top 

The Greater Washington Board of Trade is forming a joint task force with the Greater Baltimore Committee and the Economic Alliance of Greater Baltimore to promote the region as a global hotbed for the cybersecurity industry.
The Baltimore-Washington Cyber Task Force plans to work with both public and private sector groups to develop a strategy for cyber industry growth around the new U.S. Cyber Command at the Army’s Fort Meade, located halfway between Washington and Baltimore.
back to top 

React Labs, an early stage company commercializing a comprehensive mobile technology platform for real-time polling, has been awarded $100,000 in funding from the Maryland Innovation Initiative (MII), company officials announce today.
Founded by University of Maryland Professor Philip Resnik, who holds joint appointments in the Department of Linguistics and at the Institute for Advanced Computer Studies (UMIACS), React Labs offers a mobile application that allows a very large numbers of participants to register their moment-by-moment reactions to live and televised events, providing a highly engaging user experience and producing rich, detailed data for analysis.
back to top 

A team of students at the Johns Hopkins University’s Whiting School of Engineering has designed for NASA a new stethoscope that delivers accurate heart- and body-sounds to medics who are trying to assess astronauts’ health on long missions in noisy spacecraft.
Space is serene, because no air means no sound. But inside the average spacecraft, with its whirring fans, humming computers and buzzing instruments, is about as raucous as a party filled with laughing, talking people.
back to top 

Maryland was recently named the top state in the nation for entrepreneurship and innovation. The state has the second highest concentration of STEM employment and adds STEM jobs faster than all but five other states.
With Maryland’s commitment to growth in science, technology, engineering and math, UMBC continues to develop and expand its professional programs to meet the needs of the state’s STEM-centric workforce.
back to top 

The board of GenVec Inc. has voted to liquidate and dissolve the struggling Gaithersburg biotech, it announced Tuesday in a securities filing.
The move follows a string of setbacks for GenVec, which three years ago saw its lead pancreatic cancer drug candidate, TNFerade, fail in late-stage trials. In 2011, longtime CEO Paul Fischer announced his retirement from the company.
back to top 

Please join us for an exclusive, invitation-only event to be held in conjunction with PwC and the Technology Council of Maryland.
This event has been designed especially with you in mind. You’ll have the opportunity to network with your peers in an intimate setting and learn what it took companies within your industry to grow and become $1 billion market leaders. Presenter Brian Williams is a successful life sciences entrepreneur. Now consulting for PwC, Brian will present a roadmap other life science companies have followed to experience explosive growth in a dynamic and demanding environment.
Amid the volatile blend of opportunity and challenge that characterize the global life sciences industry, only a few small companies have managed to catapult their revenue over the $1 billion mark over the past two decades. Whether they chose to expand their focus and product portfolios, enter new geographies, or grow their core business, these aspiring giants pursued three distinct strategies to jump start growth:
- Leveraging core product/technology capabilities to launch differentiated products
- Using mergers & acquisitions and partnerships to gain new products and/or expand geographic presence
- Building a strong, stable leadership team armed with a compelling vision and relentless drive
We hope you can join us to explore these topics and discuss their applicability to you and your company.
When:
Tuesday, June 11, 2013
Time:
8:00 – 8:30 am – Registration and networking
8:30 – 10:30 am – Program
Location: The Universities at Shady Grove Conference Center
9630 Gudelsky Drive
Building II, Room 11-1042
Rockville, Maryland
If you have any questions regarding the event, please Deana Mary at deana.mary@us.pwc.com or 703.918.3631<
back to top 

QIAGEN N.V. (NASDAQ: QGEN; Frankfurt Prime Standard: QIA) and SIP Biotech Development Co., Ltd. (BioBAY) today announced the opening of QIAGEN (Suzhou) Translational Medicine Center, a translational medicine R&D Corporation which aims to accelerate the discovery and validation of biomarkers, and to create companion diagnostics for the Chinese market. QIAGEN (Suzhou) is a joint venture of QIAGEN and BioBAY, the innovative life sciences cluster in Suzhou Industrial Park near Shanghai. The companies announced the launch of QIAGEN (Suzhou) today in an opening ceremony on the BioBAY campus, which currently hosts more than 330 companies and research groups.
QIAGEN (Suzhou) will provide services and consulting with state-of-the-art QIAGEN molecular technologies for international and Chinese pharmaceutical companies, as well as research institutes to enable translational medicine, the multidisciplinary process of advancing discoveries from laboratory bench to the patient’s bedside. The center will work with partners located at BioBAY and elsewhere in China to provide fully integrated biomarker solutions to accelerate drug development, as well as to commercialize companion diagnostics. The four key service sections include biobanking, pharmacogenetics, next generation sequencing (NGS) and pharmacogenomics. This innovative alliance builds on QIAGEN’s leading global position in Personalized Healthcare, using genomic information to produce individualized treatment decisions for patients. QIAGEN (Suzhou) is expected to grow to about 50 employees within three years.
back to top 

It’s tough to move the needle on a multi-billion-dollar venture fund. That’s one reason New Enterprise Associates, which has managed mega-funds about as long as anyone in the business, makes a habit of taking large stakes in portfolio companies.
That strategy can pay off well in the event of a big exit – which is what happened last week with the IPO of data analysis provider Tableau Software. NEA, a backer in all of Tableau’s venture funding rounds, was the largest shareholder at the time of the offering. And as Tableau shares soared post-debut, NEA’s stake did as well. The firm sold 1 million shares in the offering for $31 million, and its remaining 18.6 million shares were worth more than $900 million as of last week.
back to top 

If you’re a medical intern, most of what you need to do your job can be pulled off a computer screen: Blood test results. Paged messages. Orders to start a medication. All but, of course, how sick a patient is.
Researchers at Johns Hopkins University and the University of Maryland, suspecting that more and more of an intern’s time is spent in front of a computer, looked into just how today’s intern spends her working hours on an inpatient ward. They asked trained college students to shadow 29 internal medicine interns from two different Baltimore teaching hospitals and document how much time they spent talking to patients, eating lunch, reading charts, and the like — over the course of three weeks. Their recently published results confirm a trend that old-timers nostalgically lament and that those of us in training know to be all too true: Only a small percentage of our time is spent in direct patient care.
back to top 

Remember Plexxikon? The Berkeley, CA-based company had a lot of talent for structural biology-based drug design, an impressive new treatment for melanoma, and a strong management team.
Two years ago, nobody on Wall Street cared one whit. Plexxikon flirted with the idea of going public, found little interest, and sold itself off to Japan-based Daiichi Sankyo for a more than 10-fold return on investment. It was a poster child for how dead the biotech IPO market was in 2010 and 2011.
back to top 

Five top Japanese drug companies are to open their “libraries” of experimental compounds to scrutiny by scientists hunting new treatments for malaria, tuberculosis and other diseases affecting the world’s poor.
The initiative, announced on Thursday, is the first project under a new $100 million partnership between the drugmakers, the Japanese government and the Bill & Melinda Gates Foundation to fund research into neglected tropical diseases.
back to top 

This week, U.S. Chief Technology Officer Todd Park and HHS Secretary Kathleen Sebelius posted separate entries on the White House Blog touting the effect of federal initiatives on health IT adoption and health industry innovation, FierceHealthIT reports.
The posts were written in response to a New York Times commentary published by columnist Thomas Friedman last week about health industry innovation related to the Affordable Care Act and other federal initiatives.
back to top 

Purpose
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and participating Institutes invite grant applications for research on the development of safe, real-time, non-invasive (or minimally invasive), in vivo methods to assess the development and function of the human placenta.
Background
The placenta is essential for the maintenance of pregnancy. The prominent function of the placenta is in the transfer of nutrients, gases and waste products between the mother and fetus. It is effectively the lung, gut, and kidney of the fetus. Abnormalities of placental development and function are known to underlie many major pathologies of pregnancy including spontaneous preterm birth, fetal growth restriction, and preeclampsia. Most information on placental biology is obtained studying placental tissue obtained from pathological pregnancies, such as a preterm deliveries occurring predominately in the third trimester, or from term deliveries in which placental development has already crested. Hence, there is a paucity of information obtained earlier in gestation, a period of time when many of the pregnancy pathologies are believed to have their origins, as well as very limited information gleaned throughout gestation from normal pregnancies. The development of real-time, non-invasive (or minimally invasive) methods to assess the development and functionality of the placenta in vivo throughout gestation would serve as valuable research tools to enhance our understanding of placental biology and rooted pathologies. The development of these tools could lead to the identification of markers and predictors of pregnancy outcome, and provide a future foundation for better pregnancy monitoring in the clinical setting.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 3- 4
OMNI Shoreham Hotel

June 5- 6
Mead Center for American Theater

June 5- 6
Sheraton Crystal City

June 6
UMBC Campus

June 11
The Universities at Shady Grove Conference Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|

BioHeaIth Innovation aims to transform Central Maryland into a commercialization hub by building an entrepreneurial ecosystem using the research, funding, networks and business development resources available in the region.
The market for Health Information Technology concepts and new products are moving at a rapid pace. Since the signing of the Health Information Technology for Economic and Clinical Health Act (HITECH) by President Obama in 2009 that allocated $36 billion to aid investments in healthcare IT and spurring new initiatives like the Office of the National Coordinator for Health Information Technology (ONC) the environment and funding available to test new products significantly escalated.
Fast forward four years—there remains to be enormous untapped market opportunity with many new products being brought to fruition and highly innovative new concepts under development by the private and public sectors.
Maryland, a significant center of the health care industry nationally and home to the National Institutes of Health, Food and Drug Administration, Johns Hopkins University, the University System of Maryland, and a robust private sector has emerging opportunities within the Health IT industry sector from telemedicine and remote monitoring to electronic medical records and mobile health.
back to top 

BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, today announced its selection of Ram Aiyar, Ph.D., M.B.A., as the first Entrepreneur-in-Residence (EIR) for BHI at the National Institutes of Health’s (NIH) National Heart, Lung and Blood Institute (NHLBI). Dr. Aiyar will help advance fundamental research discoveries to new therapeutics, diagnostics and devices that can be used clinically and commercially.
“We’re pleased to add Dr. Aiyar to our roster of Entrepreneurs-in-Residence,” said Rich Bendis, BHI President and CEO. “He is now our third EIR – joining Todd Chappell, who is EIR at NIH, and Ken Malone, our EIR working with the University of Maryland Ventures. The growth of this program will be a benefit to BHI and our partner organizations for years to come, and will result in transitioning more early-stage biomedical technologies to commercial potential. ”
back to top 

Hall of Fame award underlines long history of commitment to Maryland technology community
The Tech Council of Maryland honored Lockheed Martin with the 2013 Hall of Fame Award at its 25th annual Tech Awards Celebration, held at the Bethesda North Marriot Hotel and Conference Center in Bethesda, Md.
The Tech Awards Celebration is the mid-Atlantic region’s largest and most prestigious awards ceremony that recognizes leaders and innovators in the technology and life science communities from Maryland and the surrounding regions.
back to top 

Doug Doerfler, CEO of Gaithersburg-based MaxCyte Inc., was this month named the Tech Council of Maryland’s new chairman. He steps into the position with a wealth of TCM experience under his belt, having spent three years as chairman of the trade group’s biotech division. I caught up with Doerfler on his plans for TCM, the interplay between its IT and bio contingents and the search for a new full-time CEO.
back to top 

The threshold for reporting on marketing stunts is a high one, and United Therapeutics Corp. has just cleared it.
Reaching us by mail today is the Silver Spring biotech’s 2012 annual report, written as a spoof of a children’s book and called “Good Year UTHR.” UTHR, of course, is the ticker symbol for United Therapeutics, which has a history of doing this sort of thing.
back to top 

Medical technology pioneer AstraZeneca has chosen the Cambridge Biomedical Campus at the Addenbrooke’s Hospital site in Cambridge UK for its new global headquarters.
It will make a formal announcement in June. The Biomedical Campus has been chosen over Granta Park where AZ’s biologics division MedImmune has massively grown its presence. None of the parties involved are making any comment. The decision is a major boost for the Biomedical Campus where The Queen today officially opened the MRC’s Laboratory of Molecular Biology.
back to top 
In a unique program called Preparing the Future (PTF), 334 students have completed classroom and hands-on training to equip them to address the HIV epidemic, according to  Alexandra “Allie” Reitz, the programýs coordinator for the JACQUES Initiative (JI) of the Institute of Human Virology at the University of Maryland School of Medicine. Alexandra “Allie” Reitz, the programýs coordinator for the JACQUES Initiative (JI) of the Institute of Human Virology at the University of Maryland School of Medicine.
The PTF at the University of Maryland, Baltimore (UMB) is designed as a model for the nation and is supported by a grant from Gilead Sciencesý HIV FOCUS Program, for the JACQUES Initiative (JI). By participating, UMB students “gain invaluable communication skills through the PTF’s interprofessional approach,” says Reitz.
back to top 

GlaxoSmithKline (GSK) plc and the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services (HHS), have agreed to a first of its kind collaboration that will support the development of several antibiotics to fight antibiotic resistance and bioterrorism.
This public-private agreement marks the first time that HHS has taken a “portfolio approach” to funding drug development with a private sector company. This unique collaboration provides flexibility to move funding around GSK’s antibacterial portfolio, rather than focusing on just one drug candidate and allow medicines to be studied for the potential treatment of both conventional and biothreat pathogens.
back to top 

GlaxoSmithKline has created a contest for academic researchers that it hopes will accelerate the speed by which academic research can be turned into novel therapies.
The London pharmaceutical company, which has large operations in the Philadelphia region, on Tuesday launched a competition program it is calling Discovery Fast Track.
back to top 

The StartRight! Women’s Business Plan Competition was founded in 2003 by Rockville Economic Development Inc. (REDI) to encourage and support women’s entrepreneurship. Currently in its tenth year, StartRight! awards prizes for winning business plans annually. The women who join our competition receive more than the opportunity to win a top prize of $5,000 – they also receive valuable coaching and feedback on their business plan!
There are 3 Prize Categories – Total of $15,000 in prizes with a top prize of $5,000! You select the category in which you wish you plan to be entered.
- Technology
- General Business
- Life Science
If you have additional questions or need assistance, contact alicia@marylandwbc.org or call us at 301-315-8096.
back to top 

Biotech has never quite taken flight in Northern Virginia. Whether that’s due to the lack of a big corporate anchor or blue-chip research university, the dearth of wet labs, the attraction of a stronger scene in Montgomery County or pure dumb happenstance is anyone’s guess.
But on this side of the D.C. suburbs, the life sciences are not thriving. The story of Virginia biotech right now has much more to do with Charlottesville than it does with Fairfax.
back to top 

Wednesday, June 12, 2013 8:00 AM – 10:00 AM
Bethesda Country Club
We often hear that capital formation is among the most difficult challenges faced by biotechnology companies. Please join us on June 12th for an MdBio breakfast program to hear from four seasoned local venture capital investors, all to discuss investment trends, strategies and opportunities in Maryland. The event will offer a great platform to hear and have dialogue about the broader dynamics in investment, what has changed and where the capital opportunities are.
Speakers:
- David Mott, General Partner, NEA
- Lars Hanan, Co-Founder and Managing Partner, BroadOak
- Brian Carney, Principal, Herbert Venture Partners
- Kyp Sirinakis, Managing Partner, Rock Spring Ventures
Moderator:
- Tom Dann, Director, Maryland Venture Fund
back to top 

The Maryland Stem Cell Research Commission (Commission) has completed its review of the 171 applications received in response to its FY 2013 Requests for Applications (RFAs). The board of directors of the Maryland Technology Development Corporation (TEDCO) approved the Commission’s recommendation to fund 31 new proposals with the Maryland Stem Cell Research Fund’s (MSCRF) $10.4 million FY2013 budget. These projects, which include pre-clinical research and a clinical trial, will advance the field of regenerative medicine.
“These awards are critical to ensuring that the groundbreaking research being done has the opportunity to move to the commercial marketplace,” said Governor Martin O’Malley. “I congratulate this year’s grant recipients and look forward to the contributions they make to the improved health and wellbeing of our citizens.”
back to top 

Would it surprise you to learn that Fast Company magazine just ranked Maryland the third-most innovative state in the nation? Or that Maryland took the U.S. Chamber of Commerce’s No. 1 spot for both innovation and entrepreneurship? It’s a fact: In our state’s dynamic mix of world-class universities and professional schools, institutes for advanced research, teaching hospitals, think tanks, hubs for start-up businesses and more, there exists this mysterious, economically essential activity known as innovation.
So if we are as innovative as Fast Company and the leaders of free enterprise say we are — and I believe it’s true — we have to ask ourselves a couple of questions: How did it happen? And how can we keep it going?
back to top 

Poor maternal, infant, and child health as well as inadequate coverage of family planning remain significant global health problems facing low- and middle-income countries (LMICs) today. Despite a 47% reduction since 1990, nearly 300,000 women still die annually from causes directly related to pregnancy. The majority of these deaths are attributed to preventable obstetric complications prior to, during, and following delivery, with developing countries carrying the vast majority (99%) of the burden. Additionally, although mortality for children under five years of age has decreased from 12 million annually at the beginning of the last century (in 1900), to 6.9 million annually in 2011, the burden of these deaths now falls primarily in LMICs, with most of these deaths also due to preventable causes. In these same countries mobile phone coverage and access has become nearly ubiquitous, with the International Telecommunications Union (ITU) estimating in 2013 that the number of mobile phone subscriptions (6.8 billion) is nearly equal to the human population of 7.1 billion. The opportunity this represents is one that has not been lost on the global health community.
back to top 

June 13th – The Ritz-Carlton, Washington, DC
There is a critical unmet need in the U.S. and around the world for the development of pediatric medical devices, with support coming only from a scarce number of available grants and some private investments and philanthropy. The testing and marketing of new devices for children raise unique challenges as well. Finally, there has been much controversy around the 510(k) process for device approvals, which leads to additional need for new innovative approaches that improve the regulatory pathways for medical device development.
As we shift to a value-based healthcare system, regulatory bodies, innovators, and manufacturers must find the right balance between two noble goals: encouraging and enabling innovative medical advancements and ensuring that patients receive treatment that is as safe and effective as possible.
Please join us on June 13, 2013, for a day long symposium with leaders from the FDA, NIH, IOM, and industry, as well as policymakers, clinicians, lawyers, scientists, and bioethicists from around the world to discuss these critical issues in pediatric surgical innovation and device development.
back to top 

Our world needs entrepreneurs
Their ability to innovate, to inspire others, to power a business along the difficult journey from start-up to market leader is truly extraordinary. The Ernst & Young Entrepreneur Of The Year Award celebrates these special people who have created many of the world’s most dynamic and successful companies. We invite you to join us in celebrating their success at the Ernst & Young Entrepreneur Of The Year 2013 Maryland Awards Gala.
Wednesday, June 26, 2013
Baltimore Marriott Waterfront
700 Aliceanna Street | Baltimore, MD 21202
6:00 p.m. — Cocktail reception
7:00 p.m. — Dinner and awards ceremony
Black tie
back to top 
T oday is Demo Day for Techstars Boston. I love Techstars Demo Days for many reasons, not the least of which is the amazing community that gathers to hear the brief, well-rehearsed pitches from the various start-ups who have spent months planning for this big event. oday is Demo Day for Techstars Boston. I love Techstars Demo Days for many reasons, not the least of which is the amazing community that gathers to hear the brief, well-rehearsed pitches from the various start-ups who have spent months planning for this big event.
As accelerators like Techstars gain in popularity, many entrepreneurs wonder whether they should be applying and, if admitted, joining an accelerator and when they shouldn’t. I get this question a lot from my students, particularly as they’re graduating and scrambling to figure out where they should start their company, how to raise capital and whether an accelerator is right for them. Here are a few guidelines that I would think about if I were an entrepreneur making such a decisions.
back to top 
There are lots of myths about venture capital and biotech in particular, as noted previously on this blog. Many of these myths are deeply held beliefs about returns, what works and what doesn’t, and the state of the industry. Told often enough, these beliefs are presumed to be true by many observers, including practitioners in the field, Limited Partners, and pundits.
Surprisingly, data exists to address lots of these points, and I’ve attempted here to summarize (and link to) a number of prior posts aimed at debunking these myths and sharing a few observations on them.
back to top 

The Public Health Service Act indicates that the purpose of the National Center for Advancing Translational Sciences (NCATS) is to advance translational sciences by coordinating and developing resources that leverage basic research in support of translational science; and by developing partnerships and working cooperatively to foster synergy in ways that do not create duplication, redundancy and competition with industry activities.
back to top 
T he Affordable Care Act, aka health care reform, aka Obamacare, is spurring a massive creation of new business opportunities. he Affordable Care Act, aka health care reform, aka Obamacare, is spurring a massive creation of new business opportunities.
So says Bryan Sivak, the chief technical officer and entrepreneur-in-residence at the Department of Health and Human Services, the cabinet-level agency that regulates the $2.8 trillion U.S. health care market. Sivak joined VentureBeat’s HealthBeat conference today via a video conference (see photo above).
Just one of the areas that’s becoming fertile ground for entrepreneurial innovation: the health insurance exchanges mandated by the law.
back to top 

More than half of all eligible providers nationwide have received federal incentive payments for demonstrating meaningful use of electronic health records, rates that have more than doubled since last year alone, HHS Secretary Kathleen Sebelius announced Wednesday.
Sebelius says HHS has met and exceeded its goal for 50 percent of doctor offices and 80 percent of eligible hospitals to have adopted EHRs by 2013’s end.
back to top 

Zina Moukheiber said the New York Digital Health Accelerator Is a Model to Emulate at the beginning of the program. With the proliferation of accelerators, I thought I’d share an insider’s perspective on what it was like to be in the program now that it is complete. I’ll also share some ideas on how can take it to the next level building off of their already-strong foundation.
Zina described the program as follows:
One of the toughest hurdles for health IT start-ups is getting in front of customers. Doctors are reluctant to pay, and sales cycles at hospitals can take months. Entrepreneurs often inspired by a negative personal experience, and moved to fix the problem, find later that their product doesn’t fit the hospital’s “workflow,” or offers no incentive for doctors to adopt it.
back to top 
O ptimistic and confident in their abilities, a diverse and growing percentage of U.S. citizens engage in entrepreneurship, according to the Global Entrepreneurship Monitor (GEM). ptimistic and confident in their abilities, a diverse and growing percentage of U.S. citizens engage in entrepreneurship, according to the Global Entrepreneurship Monitor (GEM).
U.S. entrepreneurship rates climbed to the highest level in more than a decade according to the 2012 Global Entrepreneurship Monitor (GEM) U.S. Report issued today by Babson College and Baruch College. In 2012, the average Total Early-Stage Entrepreneurial Activity rate (TEA) increased to nearly 13 percent, an all-time high since GEM first began tracking entrepreneurship rates in 1999.
“Despite a sluggish economy, 2012 was marked by U.S. entrepreneurs reporting greater optimism and confidence in their abilities to start new businesses,” commented the GEM Report’s lead author, Donna J. Kelley, Associate Professor of Entrepreneurship at Babson College. “In fact, nearly 13 percent of the U.S. adult population was engaged in entrepreneurship with the vast majority starting businesses to pursue an opportunity rather than out of necessity. On the downside, Americans closing businesses were twice as likely as those in other innovation-driven economies to cite difficulties financing their ventures.”
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 3- 4
OMNI Shoreham Hotel

June 5- 6
Sheraton Crystal City

June 6
UMBC Campus
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:
 |
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, today announced its selection of Ram Aiyar, Ph.D., M.B.A., as the first Entrepreneur-in-Residence (EIR) for BHI at the National Institutes of Health’s (NIH) National Heart, Lung and Blood Institute (NHLBI). Dr. Aiyar will help advance fundamental research discoveries to new therapeutics, diagnostics and devices that can be used clinically and commercially.
“We’re pleased to add Dr. Aiyar to our roster of Entrepreneurs-in-Residence,” said Rich Bendis, BHI President and CEO. “He is now our third EIR – joining Todd Chappell, who is EIR at NIH, and Ken Malone, our EIR working with the University of Maryland Ventures. The growth of this program will be a benefit to BHI and our partner organizations for years to come, and will result in transitioning more early-stage biomedical technologies to commercial potential. ”
back to top 
 POSITION DESCRIPTION – Health IT Entrepreneur-in-Residence POSITION DESCRIPTION – Health IT Entrepreneur-in-Residence
The Health IT Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage health IT technologies, advise BHI on opportunities for new ventures, and build a portfolio commercially relevant health IT opportunities. The Health IT EIR influences the BHI organization by managing and providing information, intelligence and insights that drive critical business decisions. The Health IT EIR will work with early stage companies to launch and validate those companies while providing recommendations and insights on the direction of potential technologies. The Health IT EIR has the potential to also serve in a co-management role in a health IT accelerator.
back to top 
 The award luncheon will provide countless opportunities to show your appreciation for the estimated 33,000 small businesses of Montgomery County—businesses that contribute directly to the strength of the area’s economy. Eight awards will be presented to eight distinct companies. The award luncheon will provide countless opportunities to show your appreciation for the estimated 33,000 small businesses of Montgomery County—businesses that contribute directly to the strength of the area’s economy. Eight awards will be presented to eight distinct companies.
Registration and networking with exhibits will begin at 11:00 AM, followed by the program at 12:00 PM./p>
May 24
Mariott Bethesda North Conference Center
back to top 

America urgently needs a national, research-based effort to empower all undergraduates and help more of them, particularly underrepresented minorities, graduate with science and engineering degrees, said Freeman Hrabowski, III, president of the University of Maryland, Baltimore County (UMBC) during the 2013 William D. Carey lecture.
Hrabowski addressed the 38th Annual AAAS Forum on Science and Technology Policy on 2 May 2013—fifty years to the day after he had participated in the historic Birmingham Children’s March, which was inspired by the late Dr. Martin Luther King, Jr. “I wanted a better education,” Hrabowski said of his participation in that 1963 event. “All children really do want to be well-educated.”
back to top 

The Economic Alliance of Greater Baltimore and the Maryland Department of Business of Economic Development announced Monday that they created a new program to help companies that have moved beyond the start-up phase to continue to grow.
Advance Maryland is designed to assist these companies with developing markets, fine-tuning their business models and boosting growth with the help of a research specialist. Similar models have been adopted in other states, the groups said.
back to top 
Tuesday, June 11, 2013, 05:00pm – 06:30pm
 Please join us for an exclusive MedTech networking event hosted by QIAGEN. Learn more about AdvaMed 2013: The MedTech Conference and discover your local MedTech community. Please join us for an exclusive MedTech networking event hosted by QIAGEN. Learn more about AdvaMed 2013: The MedTech Conference and discover your local MedTech community.
Please RSVP by June 3 to Lauren Goldstein at
lauren@medtechconference.org
This email address is being protected from spambots. You need JavaScript enabled to view it.
or 202.434.7213
Location QIAGEN’s Germantown Facility
back to top 

The Tech Council of Maryland (TCM), the state’s largest technology trade association with more than 400 biotechnology and technology members employing more than 200,000 in the region, announced at its 25th annual Dinner and Awards Celebration last night that Doug Doerfler was elected as chairman of TCM’s board. Doerfler was the association’s vice chairman and succeeds Larry Letow, who has served as chairman since 2010. TCM also named four current board members to executive leadership positions.
“I am honored to continue serving TCM at such an important and promising time,” said Doerfler. “Our members have become the catalysts of Maryland’s dynamic economy, creating new, high-paying jobs and discovering breakthrough technologies that can change our world. We may come from diverse backgrounds, but a single mission unifies us: creating a healthier, safer world with game-changing innovations right here in Maryland. I especially thank Larry Letow for his outstanding board leadership these past three years. He has positioned TCM, our membership and Maryland for a very bright future.”
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association with more than 400 biotechnology and technology members employing more than 200,000 in the region, last night announced the winners of its 2013 TCM Awards. The 25th annual celebration was attended by more than 750 technology and business leaders from around the state.
“Maryland is ripe with innovative companies – from ones focused on high tech and IT solutions that make businesses and governments run smoothly and securely, to biotech companies that are developing cutting-edge cures for serious diseases,” said Doug Doerfler, chairman of TCM’s Board and founding president and CEO of MaxCyte, Inc. “Winners of the 2013 Tech Awards exemplify this broad cross section of individuals and companies that make up the Tech Council membership and are key to driving advances in all aspects of technology.”
back to top 

As a follow up to a $1 billion initiative last year that funded projects across the nation designed to improve outcomes and save money in the healthcare system, the Centers for Medicaid & Medicare Services today announced a second, $1 billion round of Health Care Innovation Awards.
According to CMS, funding will be awarded to provider groups, health systems, payers, states, public-private partnerships, for-profit organizations or any other parties that have developed innovative payment and delivery models to improve population health, quality of care and cost efficiency.
back to top 

Fifty years ago this month, I chanced to hear the Rev. Martin Luther King Jr. I was a mild-mannered kid with a speech impediment and a love of math. That day, I was focused on solving math problems, not issues of justice and equal rights. But King broke through to me when he said this: If the children of Birmingham march, Americans will see that what they are asking for is a better education. They will see that even the very young know the difference between right and wrong.
I chose to march, and found myself among hundreds of children jailed for five terrifying days. Mind you, I was not a brave child. But even at 12 years old, I believed and hoped that my participation could make a difference.
back to top 

A District advisory committee OK’d bids from Johns Hopkins Medicine and MedStar Health to build costly proton therapy centers on Thursday, leaving both just one step from final approval to proceed on the high-tech cancer treatment programs.
It wasn’t, however, a clean victory for either. The committee rejected a key part of Hopkins’ plan and asked for additional details about both nonprofit system’s outreach to under-served neighborhoods.
back to top 
Having recently stressed that oncology is a core therapy area for its research, AstraZeneca is moving three of its cancer compounds into Phase III trials.
First up, MedImmune, AstraZeneca’s biologics R&D arm, has enrolled the first patient in a late-stage study of moxetumomab pasudotox. It is sponsored by the Cancer Therapy Evaluation Program at the US National Cancer Institute (NCI), and will evaluate the CD22 immunotoxin as a potential treatment in adults with hairy cell leukaemia who have not responded to or relapsed after standard therapy.
back to top 

Entrepreneur Magazine has named Maryland the No. 1 state to start a new business, with neighboring Virginia following close behind at No. 3.
The California-based magazine cited the state’s Maryland Entrepreneurs Resource List — which connects experts with startups — and the University of Maryland Baltimore County’s initiative to find and train female entrepreneurs in choosing the state for the top position.
back to top 
By Eve Green

Last week, the Baltimore BioPark played host to the Innovation Working Group, which consists of executives from the U.S.-Russia Bilateral Presidential Commission. The commission was founded as a joint venture between President Barack Obama and Russian President Dmitry Medvedev.
The highly anticipated event is an opportunity for the United States and Russia to find new ways to collaborate on projects in the fields of biotech and science. Members of the group were given a tour of the University of Maryland, College Park and Baltimore BioPark. The event was led by Oleg Fomichev, the Russian Deputy Minister of Economy, and Lorraine Hariton, Special Representative for Business and Commerce of the U.S. Department of State. Among those that joined the three day tour were chief executives of biotech companies from both Maryland and Russia, as well as leaders from the Pushchino BioTech Cluster.
back to top 
Telehealth holds enormous potential for transforming healthcare, but, to telemedicine pioneer Dr. Jay Sanders, the primary barrier is not financial. Instead, physician and patient attitudes about healthcare and health itself must change, said Sanders, who often has been called the father of telemedicine.
“The critical issue is really not telemedicine,” Sanders said in an interview with MobiHealthNews, noting that the term can include telehealth, mobile health, digital health and other forms of e-health. “What’s important is the needs of the healthcare delivery system.”
back to top 

AURP 2015 International Conference Host
Would you like to highlight your city and your university research park by hosting AURP’s 2015 International Conference? As the host park, you’ll be recognized as a leader in the field and attract worldwide attention to the exciting progress and success of your region. Find out more about the requirements at AURP.net/hostrfp.
To be considered, submit your proposal, electronically, to VickiePalmer@AURP.net.
Deadline: Friday, September 6, 2013.
back to top 

Chevy Chase-based New Enterprise Associates stands to reap a fortune from Tableau Software Inc.’s newly public stock, which is up about 60 percent in its first day of trading on the New York Stock Exchange Friday.
NEA is the single biggest venture backer of the Seattle-based data analytics company (NYSE: DATA). The firm sold 1 million shares of Tableau stock in the offering, and is holding on to another 18.5 million shares, or about 37 percent of the company. With Friday’s pop, that stake is valued at more than $900 million.
back to top 

“Transformation” is the best description of what is happening in health care right now. We are seeing historic changes in how health care is administered in the United States—with increased focus on quality of care versus just paying for a service. We are seeing changes in how people can enroll in health insurance—with the upcoming establishment of a new market place that will help more people get insured in this country than ever before. And, we are seeing changes in how people understand and make decisions about their own health—with an increasing number of tools and services becoming available to help individuals access health information and manage their own personal health data.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

May 21
Online Webinar
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|

Local businesses have seen needed venture capital and loans in greater abundance in recent months.
The amount of venture capital investment funneled to Montgomery County and Washington-area companies in 2013’s first quarter jumped by 30 percent from the same period a year ago, according to a recent report.
The $286.3 million invested in local companies in the first three months was the most in the quarter in five years, according to the report from PricewaterhouseCoopers LLP and the National Venture Capital Association, based on data by Thomson Reuters.
back to top 

It’s true that, geographically speaking, Maryland is a small state. But as the old saying goes, “Good things come in small packages,” and that is especially true when it comes to the resources, advice and technical expertise that Maryland offers inventors and technology entrepreneurs.
From university laboratories and research centers (such as The Johns Hopkins Applied Physics Laboratory, University of Maryland’s Office of Technology Commercialization or University of Maryland – Baltimore City’s ACTiVATE) to state organizations (Maryland Biotechnology Center or the Department of Business and Economic Development), a wide variety of entities are working to help entrepreneurs develop and market their inventions.
back to top 

Benlysta, a drug to treat lupus, has received approval from European regulators to be marketed in Europe, the drug’s co-makers, Human Genome Sciences Inc. of Rockville, and GlaxoSmithKline, its London-based parent, announced.
The European approval came two days after Benlysta, the first new treatment developed for lupus in 50 years, was approved for use in Canada.
back to top 

The University of Maryland School of Medicine is launching a $500 million fundraising campaign, the largest in the school’s history.
The school plans to officially launch the capital campaign, “Transforming Medicine Beyond Imagination,” at its annual gala May 11. The event is considered a critical fundraiser for the school’s research and clinical programs.
back to top 

Pluristem Therapeutics Inc. (Nasdaq:PSTI) (TASE:PLTR) today announced that its wholly owned subsidiary, Pluristem Ltd., has entered into an exclusive out-license agreement with United Therapeutics Corporation (Nasdaq:UTHR) for the use of Pluristem’s PLacental eXpanded (PLX) cells to develop and commercialize a cell-based product for the treatment of Pulmonary Hypertension (PH).
Under the terms of the agreement, United Therapeutics will receive exclusive worldwide licensing rights for the development and commercialization of the future product for treating PH patients. Pluristem will retain all manufacturing rights; participate in the pre-clinical and clinical trial activities, as well as provide the commercial grade product.
back to top 

A Johns Hopkins University faculty member will be helping advise the National Institutes of Health about research topics with the most promise for addressing public health challenges.
Janice E. Clements, vice dean for faculty at the Johns Hopkins University School of Medicine, has been selected to serve on the Council of Councils for NIH. Clements was among 10 individuals newly selected to serve on the 27-member council. Her term ends in October 2015.
back to top 

UK pharmaceuticals giant GlaxoSmithKline has unveiled an “open innovation” platform through which it will publish data from its clinical trials online so it can be analysed by external researchers.
The online system allows scientists to submit a to request access anonymised drug trial data. Each request will be judged a panel of external experts appointed by GSK, and applicants must agree to publish their own findings.
back to top 

Thursday, May 23rd at 5:00 p.m.
Join NFTE Baltimore as an honored guest at out Regional City Wide Business Plan Competition. The top size finalists will present and defend their business plans before a prestigious panel of judges and an audience of top buiness and school leaders. The networking reception will include a Student Showcase and we will also honor NFTE’s biggest supporters of youth entrepreneurship.
University of Baltimore
William T. Thumel Sr. Business Center
11 West Mount Royal Avenue
Seating is limited RSVP here by May 20th.
back to top 

May 15, 2013 8:00am – 10:00am
Tower Club, Tysons Corner
Attracting and retaining key employees is a challenge every business faces, and government contractors know that a valuable contract can hinge on making the right staffing choices. Savvy government contractors also recognize the necessity to maximize human capital within the constraints of sequestration, continuing resolution and lowest price technically acceptable contracts.
You’re invited to this breakfast workshop to learn about powerful employee incentive compensation strategies that can provide significant competitive advantages for your business. These often underutilized plans can attract top quality employees through cash, equity and other equity-linked compensation methods. In addition to creating great work environments, innovative incentive plans allow government contractors to be successful amidst the current era of regulatory challenges.
back to top 

Venture capital firm Grotech Ventures has raised $225 million that it plans to invest in early-stage technology companies.
The $225 million round, called Grotech Ventures II, is the firm’s eight fund. The firm had originally planned to raise $220 million but expanded to include new investors, said Grotech Managing General Partner and Founder Frank Adams.
back to top 

Governor Martin O’Malley today officially launched data.maryland.gov – Maryland’s first statewide open data portal that will provide researchers, entrepreneurs, public servants and citizens with a wide variety of data to support transparency and innovation in government. Data such as vendor payments, vehicle accidents, licensed veterinary clinics, GIS mapping data, and per capita electricity consumption will all be made available and housed in a central place for the public.
The Governor made this announcement at a panel discussion hosted in conjunction with the Future of Information Alliance (FIA) – a transdisciplinary partnership between the University of Maryland, College Park and 10 founding partners. The panel featured “futurists” and over 120 students, entrepreneurs and public servants who spoke about the importance of big data to better serve and inform the public.
back to top 

As the provisions of the Affordable Care Act and HITECH Act are rolled out, providers and payers are looking for ways to address pain points varying from improving patient engagement and remote monitoring to helping consumers better understand their insurance options to making better use of patient information in the context of big data.
Some accelerators are helping members of the healthcare ecosystem and entrepreneurs find each other. These four healthcare accelerators are currently looking for applicants.
back to top 

Version 2.0 of a plan to create an Office of mHealth in the U.S. Food and Drug Administration should be out soon. The office of U.S. Rep. Mike Honda, Silicon Valley’s Democratic Congressman who authored the legislation, said the next draft will at the least fine-tune the way the office would work with other federal agencies.
But is the big-ticket item – a new office dedicated to adding more context and understanding of mHealth within the federal agency – the most interesting thing about the bill? I don’t think so. Read through the details and see if you agree.
back to top 

The University City Science Center is collaborating with the Canadian Consulate General to pilot a business accelerator for health information and communication technology companies from Canada.
Opening on May 13, 2013, the Canadian Technology Accelerator at the Science Center will provide a 3-6 month “market immersion” experience for Canadian health IT companies, leveraging the Science Center’s resources and networks through its Port business incubator.
back to top 

If you’re a startup working on a product for the healthcare industry, Boston is the place to be. During a trip to Bean Town, I was curious to hear what startup founders found most challenging in a city so rich with resources. The usual gripes I usually hear elsewhere, like troubles with seed funding or FDA regulation, didn’t come up as much. Instead, these founders seemed to find the more personal elements of entrepreneurship most challenging. Many times, I found myself talking to people who had made a shift from working in tech or research to becoming a business owner.
The community realizes that, though. In addition to numerous startup accelerators and incubators, Boston is home to lots of collaborative startup space.
back to top 

Venture capitalists are pushing to allow more highly educated immigrants into the U.S., saying this is essential to ensuring that the country remains an innovation leader.
One argument they use is that immigrants create jobs by starting companies. Scott Sandell, general partner at New Enterprise Associates who leads the venture firm’s technology investing, wants to support this assertion with better data.
back to top 
 I had the privilege to meet with and speak to the local Healthcare meet-up recently (thanks to Pete Kanee at www.healthcare.mn) and as part of my discussion, I shared a list of things that I’d encourage the local (healthcare) startups to continue to keep in mind as they grow their companies. I had the privilege to meet with and speak to the local Healthcare meet-up recently (thanks to Pete Kanee at www.healthcare.mn) and as part of my discussion, I shared a list of things that I’d encourage the local (healthcare) startups to continue to keep in mind as they grow their companies.
These are relevant to not only healthcare-focused startups but all startups for that matter. They’re pretty common, but in my recent conversations with healthcare entrepreneurs, I found many of these needing to be brought up as part of the dialogue.
back to top 

Health and Human Services Secretary Kathleen Sebelius is asking companies for financial donations to help implement President Barack Obama’s healthcare overhaul, months before it is due to take effect.
In telephone calls that began around March 23, officials say, Obama’s top healthcare adviser has been seeking assistance from companies in the healthcare field and other industries as well as from healthcare providers, patient advocacy groups, churches and other charitable organizations.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:
 |
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
|

Ingenuity Systems, a 15-year veteran of the biological software business, showed today that you can make money not just by generating DNA data, but by helping scientists figure out what it means.
Redwood City, CA-based Ingenuity said today it has agreed to be acquired by Netherlands-based Qiagen for $105 million in cash. Ingenuity, a private company, was able to fetch that price after it closed last year with about $20 million in net sales, the companies said in a statement. The deal is expected to start adding to Qiagen’s profits in 2015, the companies said.
back to top 

Date: Wednesday, May 8th, 2013 9:30am-5:00pm
Location: Morgan, Lewis & Bockius Conference Center, 1111 Pennsylvania Ave., NW Washington, DC 20004-2541
National Brain Tumor Society is bringing together brain tumor researchers and industry executives to discuss how to move research from bench to bedside.
- Biophrama executives and VC investors will share their criteria for investment and licensing of academic research.
- Panelists include:
- Lauren Abrey
Global Development Team Lead, Genetech/Roche
- Neil Exter
Partner, Third Rock
- Brian Gallagher
Partner, SROne
- Michael Glutch
Managing Director, Medimmune Ventures
- Perry Nisen
Senior VP of Science Transactions, Pfizer
- Nation Brain Tumor Society-funded researchers will present brain tumor drug candidates ready for early stage investment and/or licensing.
back to top 

The Maryland Technology Enterprise Institute (Mtech) today announces the winners of the inaugural University of Maryland Business Model Challenge.
The two winning UMD entrepreneur teams were selected from among 44 initial entries and 11 finalist teams, six of whom were selected to present the results they achieved through the Challenge’s multi-week lean startup workshop to an expert panel of judges on April 26 at the University of Maryland.
back to top 

Late last week, leaders from across the global vaccine industry gathered at the 6th Annual Vaccine Industry Excellence (ViE) awards ceremony during the World Vaccine Congress in Washington, D.C, to honor the industry’s best and brightest. Immunovaccine is proud to announce that chief executive officer John Trizzino was awarded this year’s Best Biotech CEO prize during the ceremony.
In considering candidates for the Best Biotech CEO award, judges evaluated a number of important criteria including:
- Degree of outstanding commitment to disease prevention and treatment
- Level of exemplary leadership throughout the year
- Contribution to company performance, communication and vision
- Influence within the industry
- Achievements made with regards to company positioning and status
back to top 

The Ernst & Young Entrepreneur Of The Year 2013 finalists have a lot to celebrate. They’ve built their dream companies. Expanded. Innovated. And now Ernst & Young is recognizing them for their achievements. Help us congratulate these outstanding entrepreneurs. Join us at this years event and see who will take home top honors.
back to top 

Johns Hopkins says it is working on its largest-ever fundraising campaign, which aims to raise $4.5 billion by the end of 2017.
The university and health system announced the effort Saturday. The idea is to create as many as 300 endowed professorships and generate nearly $700 million for undergraduate financial aid and graduate student fellowships.
back to top 

TOPIC:
Leveraging USDA-ARS Partnerships and Capabilities to Help Your Business
PRESENTERS:
Robert Griesbach, Ph.D.
Deputy Assistant Administrator Office of Technology
James Poulos, III
Technology Transfer Coordinator
Cathy Cohn
Technology Transfer Liaison
Abstract:
Agricultural Research Services (ARS) is the research arm of the Department of Agriculture that develops and transfers solutions to agricultural problems affecting Americans every day, from field to table. To accelerate the development of new products and help move technologies into the marketplace, ARS often partners with companies and research institutions early on in the research process. Working collaboratively alongside ARS, partners are able access and implement ARS technologies, resources and expertise to further develop specific products and create new ones. ARS conducts research in 800 projects at 90 locations including six pilot plants and six human nutrition centers. With a long standing commitment to technology transfer, ARS works closely with the private sector to ensure research outcomes are adopted. Please join us to learn about the wide range of expertise and capabilities from ARS that can be used to grow your business
back to top 

Emergent BioSolutions Inc. (NYSE:EBS) announced its participation in the Defence against Terrorism Programme of Work (DAT POW) workshop conducted by the North Atlantic Treaty Organization (NATO) Emerging Security Challenges Division at the Counter Terror Expo in London. An initial session of the workshop was focused on the future capabilities and protection of the Special Operations Forces (SOF) community on areas such as Improvised Explosive Devices (IEDs), Intelligence, Surveillance, Reconnaissance (ISR), Biometrics and Forensics, Information Sharing Systems, Tactical Computer, Communications, Command and Control (C4), and Force Protection.
“Emergent strongly supports NATO’s initiatives to educate on protecting those who protect the population,” said Allen Shofe, Executive Vice President of Corporate Affairs, Emergent BioSolutions. “NATO DAT POW workshops are highly relevant and informative as they cover various activities undertaken and coordinated by NATO countries.”
back to top 

The University of Maryland Institute for Bioscience and Biotechnology Research (IBBR) invites you to participate in the 5th Biennial NMR Day symposium on Friday, May 17, 2013, at our facility in Rockville, Maryland.
IBBR (formerly CARB) hosts a biennial meeting, designed to promote discussion and collaboration among scientists active in the application of biomolecular NMR to modern problems in structural biology and biotechnology. The symposium speakers are internationally recognized in the application of NMR to biological systems
back to top 

The National Capitol Area Local Chapter of SoPE in concert with JHU Carey Business School, MedChi, Zanvyl Krieger School of Arts and Sciences Center for Biotechnology Education, Montgomery County Medical Society, and the Medical Society of Northern Virginia presents:
“Working with Industry in the Life Sciences – What does it Really Take?”
Tuesday, May 14th, 2013–6:00PM to 8:00PM
Johns Hopkins University, Montgomery County Campus, Building III – 9605 Medical Center Drive, Room 121
back to top 

Cyber – Health – Mobile
Thursday, June 6, 2013 8:00am – 1:00pm —UMBC
Join us as we bring together top leaders and innovators in the fields of Cybersecurity, Health IT, and Mobile Technology. Learn where the technology is leading these 3 sectors and the opportunities that exist for real estate brokers and developers who want to capitalize on these expanding industries.
Hear from leaders including Dr. Freeman Hrabowski, one of Time Magazine’s “World’s 100 Most Influential People”, as well as Dr. Mark Doms, Under Secretary for Economic Affairs with the United States Department of Commerce, who serves as a top economic advisor to the Obama Administration and to the Department of Commerce.
back to top 

Startup Maryland today announced dates (September 9 – 27) and the rough route for the Pitch Across Maryland 2.0, the second annual state-wide tour and celebration of entrepreneurship and startup companies.
Referred to by CBS News affiliate WBOC-TV 16 as “Opportunity on Wheels,” the inaugural Pitch Across Maryland tour was an overwhelming success last year. The bus traversed the state all in the name of celebrating entrepreneurship. A sampling of details, key data and results from last year’s tour follow:
back to top 

The Johns Hopkins Bloomberg School of Public Health has partnered with Barclays Bank to develop a youth entrepreneurship program with the Bloomberg School’s Center for American Indian Health. The new initiative is aimed at designing an evidence-based program to inspire American Indian youth to stay in school and create business and social entrepreneurship opportunities.
As part of the partnership, Barclays will provide a total of $1.2 million in program funding over the course of the next three years. Additionally, Barclays employees will lend their business expertise and serve as mentors to program participants. Program efforts will first concentrate on the White Mountain Apache Reservation in Arizona with the ultimate goal to implement youth entrepreneurship programs in poverty stricken communities throughout the world.
back to top 

Earlier this month, the National Venture Capital Association (NVCA), a trade association representing the U.S. venture capital industry, released the results of its MoneyTree Report on venture funding for the first quarter of 2013. The report, which is prepared by NVCA and PriceWaterhouseCoopers LLP using data from Thomson Reuters, indicates that venture capitalists invested $5.9 billion in 863 deals in the first quarter, which constituted a 12% decrease in dollars and a 15% decrease in deals as compared with the fourth quarter of 2012, when $6.7 billion was invested in 1,013 deals (see chart below; data from MoneyTree Reports).
back to top 

Somewhere between venture funding and bank loans is a kind of investment that seems to fly under the radar, but apparently over the last several years has become increasingly popular for biotech companies beyond the startup stage looking for growth capital.
Royalty financing is the vehicle that Capital Royalty L.P. will use to invest its new $805 million fund in healthcare products and technologies. But (sorry startups), it’s not looking for early-stage companies. Capital Royalty says it invests in companies with FDA-approved healthcare products that are generating revenue. These are companies looking to make acquisitions, expand into new markets or develop new products with investments of $20 million to $200 million.
back to top 
Chandra Duggirala, maker of an experimental device for type two diabetes, is on the verge of giving up.
Duggirala’s company, Novobionics, raised a small amount of funding for a noninvasive technology that mimics the effects of gastric bypass surgery. The device tricks the gastro-intestinal tract into thinking it is full, which slows the rate of nutrient absorption, thereby easing suffering for diabetes patients.
back to top 

President Barack Obama dropped in on the National Academy of Sciences today to help it celebrate its 150th birthday.
The president said he’s committed to increased public investment in scientific research, contending this is necessary in order for the U.S. to retain its technological edge. Under sequestration, however, federal spending on research is being cut, not increased.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

May 7- 8
Sheraton Crystal City

May 8
Morgan, Lewis & Bockius Conference Center

May 12-16
Gaylord National Hotel & Convention Center

May 13-16
Gaylord National Hotel & Convention Center

May 14
Johns Hopkins University, Montgomery County Campus
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
 BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, today announced its selection of Ken Malone, Ph.D., of Early Charm Ventures, as the first BHI Entrepreneur-in-Residence (EIR) assigned to work directly with UM Ventures. Dr. Malone, a serial entrepreneur who has founded or been an officer of five biotechnology or advanced materials companies, will help advance the commercialization efforts of new start-up companies based upon innovative discoveries coming out of research programs at the University of Maryland (UMD) College Park and Baltimore. BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, today announced its selection of Ken Malone, Ph.D., of Early Charm Ventures, as the first BHI Entrepreneur-in-Residence (EIR) assigned to work directly with UM Ventures. Dr. Malone, a serial entrepreneur who has founded or been an officer of five biotechnology or advanced materials companies, will help advance the commercialization efforts of new start-up companies based upon innovative discoveries coming out of research programs at the University of Maryland (UMD) College Park and Baltimore.
“We’re thrilled to name Ken Malone as an EIR,” said Richard Bendis, BHI President & CEO. “Ken and I have a long history of working together, so I know first hand that he has the experience, drive and perspective to be a true asset to BHI and to the many start-ups he’ll serve from University of Maryland in support of the newly launched UM Ventures initiative.” Dr. Malone will assist any interested UM start-ups once they have been launched.
back to top 

Date: Wednesday, May 8th, 2013 9:30am-5:00pm
Location: Morgan, Lewis & Bockius Conference Center, 1111 Pennsylvania Ave., NW Washington, DC 20004-2541
National Brain Tumor Society is bringing together brain tumor researchers and industry executives to discuss how to move research from bench to bedside.
- Biophrama executives and VC investors will share their criteria for investment and licensing of academic research.
- Panelists include:
- Lauren Abrey
Global Development Team Lead, Genetech/Roche
- Neil Exter
Partner, Third Rock
- Brian Gallagher
Partner, SROne
- Michael Glutch
Managing Director, Medimmune Ventures
- Perry Nisen
Senior VP of Science Transactions, Pfizer
- Nation Brain Tumor Society-funded researchers will present brain tumor drug candidates ready for early stage investment and/or licensing.
back to top 

Time-reversal techniques for optimizing broadband communication networks and rapid prototyping of microfluidics devices were among the Clark School of Engineering inventions recognized as the most promising new technologies at the University of Maryland Invention of the Year Awards.
The University of Maryland’s Office of Technology Commercialization (OTC) hosted the 26th Annual Invention of the Year Awards reception on Tuesday, April 16, 2013 from 4:30-6:00 pm at the University of Maryland Golf Course Club House.
back to top 

The Biotechnology Industry Organization (BIO) is pleased to announce the election of Rachel King, President and CEO, GlycoMimetics, Inc, as the new Chair of its Board of Directors for the 2013-2014 term, and the election of David Pyott, Chairman, President, & CEO, Allergan, Inc., as its new Board Secretary. BIO also is pleased to announce the re-election of Mark Skaletsky, Chairman & CEO, Fenway Pharmaceuticals, Inc., as Board Treasurer, and the election of 19 Directors to serve on BIO’s Board Executive Committee for the new term. In addition, BIO welcomes the election of eight new members to its Board of Directors, voted upon at this year’s 2013 BIO International Convention.
“Rachel King brings a depth of industry experience and passion for advocacy that will serve BIO and its members well,” said Jim Greenwood, BIO President & CEO. “I look forward to working closely with Rachel and our newly-constituted Board of Directors in the years to come.”
back to top 

Emergent BioSolutions Inc. (NYSE: EBS) announced today that it has entered into an asset purchase agreement to acquire the Healthcare Protective Products Division (HPPD) of Bracco Diagnostics Inc. in an all-cash transaction that includes payment of $26.0 million upon closing. The acquisition will diversify and expand Emergent’s biodefense franchise by adding product sales from HPPD’s marketed chemical countermeasure, RSDL (Reactive Skin Decontamination Lotion). The acquisition offers Emergent an opportunity to leverage its core capabilities in manufacturing, government contracting, government sales, and product distribution as it looks to substantially expand sales of RSDL in the attractive and growing chemical countermeasure market.
“This acquisition directly supports our ongoing growth plan, which includes acquiring revenue generating, profitable products and businesses that address the needs of U.S. and allied foreign governments across the CBRN spectrum,” stated Daniel J. Abdun-Nabi, president and chief executive officer of Emergent BioSolutions. “Our relationships, combined with those of HPPD, with the U.S. Government and foreign ministries of defense, as well as worldwide distributor relationships addressing first responder markets, should enable us to grow revenues from RSDL and to expand and enhance Emergent’s leadership position as a premier supplier of CBRN countermeasures.”
back to top 

Last year AstraZeneca’s ($AZN) MedImmune did about 20 biologics deals, not counting the academic pacts it assembled. This year, MedImmune R&D chief Bahija Jallal tells me, there will be no letup in talent scouting. “If we do as little or more than last year,” says Jallal, “I would be happy.”
Echoing late-stage R&D chief Briggs Morrison–who spoke with me at BIO last week–Jallal singled out cardiovascular/metabolics, respiratory and cancer as key fields for new deals. She noted particular interest in brown fat, a focus for weight-loss fans, and immunotherapeutics, which has emerged as one of the hottest sectors in oncology R&D.
back to top 

ETC, Baltimore City’s technology innovation center, announced today that it has chosen a new Baltimore City location to house it’s award winning technology incubator when its current Canton lease expires in October 2013. The ETC will move to 101 N. Haven Street, an adaptive reuse of an historic industrial building that was once home to the King Cork and Seal Company. The recently renovated building is located near Highlandtown with easy access to I-95 and is within the Enterprise Zone and a HUBZone. The ETC will maintain space in its other location at Johns Hopkins University – Eastern Campus on 33rd Street.
“The Emerging Technology Center has championed small business development and job creation in Baltimore for years, and we are proud to have supported their efforts,” said Baltimore Mayor Stephanie Rawlings-Blake. “We wish them the best of luck and success in their new location. I’m confident that the ETC will continue to be an important driver of innovation and entrepreneurship in the city. ”
back to top 

Maryland often has been at the forefront of innovation with a longstanding reputation for investing in science and technology to capture new opportunities for economic growth. This year’s legislative session was no different. Lawmakers backed Gov. Martin O’Malley’s proposals to support the state’s bioscience sector, expand the R&D tax credit, enhance workforce training, and promote measures to establish the state as a leader in cybersecurity.
back to top 

I wanted to share some data with you that illustrates the strength of Montgomery County’s business community: total employment in the County grew by nearly 25,000 jobs – from 631,000 jobs in 2010 to 655,800 jobs in 2012! This is impressive given the economic climate and shows the resiliency and fortitude of our local businesses – large and small.
The top job gaining sector was professional, scientific and technical services, which added almost 5,300 jobs beteen 2010 and 2012. The other top growing sectors were government, retail trade and health care and social assistance, each adding over 3,000 jobs during the three year period.
back to top 

MedImmune has acquired Ann Arbor-based AlphaCore Pharma, creating another exit for a local up-and-coming start-up.
MedImmune, the global biologics research and development arm of AstraZeneca, has not disclosed the acquisition price nor its intentions on whether to keep the start-up in Tree Town. Tracy Rossin, director of corporate public relations for MedImmune, did write in an email that the company does “not have plans to expand its operations/workforce in Ann Arbor.” She does add that her firm is “planning to incorporate AlphaCore Pharma into the larger AstraZeneca organization.”
back to top 

What if you could create a biotech startup focused on treating a rare disease, with a drug candidate already in hand, and high odds of success in clinical trials?
That’s the concept that crystallized in former MedImmune executive David Mott’s mind through decades of experience in the life sciences sector. The idea ultimately led him to start a Cambridge, MA-based biotech incubator called Cydan. This new organization, formally announced this month, has been staffed with a hand-picked squad of specialists tasked with churning out a lineup of small companies that make drugs for orphan diseases.
back to top 

The purpose of the NHLBI SBIR Phase IIB Bridge Award is to facilitate and accelerate the capital-intensive steps that are required to transition SBIR Phase II projects to the commercialization stage by promoting partnerships between SBIR Phase II awardees and third-party investors and/or strategic partners. The Bridge Award encourages business relationships between applicant small business concerns and third-party investors/strategic partners who can provide substantial financing to help accelerate the commercialization of promising new products and technologies that were initiated with SBIR funding. In particular, applicants are expected to leverage their previous SBIR support, as well as the opportunity to compete for additional funding through the NHLBI Bridge Award program, to attract and negotiate third-party financing needed to advance a product or technology toward commercialization. The applicant’s ability to secure independent third-party investor funds that equal or exceed the total amount of the NHLBI funds being requested over the entire Bridge Award project period will help to validate the commercial potential that is essential for the SBIR projects solicited under the Bridge Award program. It is anticipated that many of the partnerships between small businesses and third-party investors will involve a considerable level of project due diligence by the private sector, thereby increasing the likelihood of commercial success for the funded projects. In light of these goals, the NHLBI strongly encourages applicants to establish business relationships with investors, strategic partners or both that have appropriate prior experience in commercializing emerging biomedical technologies.
back to top 

Holding the keys to the future of health care and medicine is not something many children dream of doing.
But Bahija Jallal, executive vice president of AstraZeneca’s (AZN) biologics arm, MedImmune, knew from a very young age that was the career she was destined for.
back to top 

The University of Maryland has signed an agreement for a student-exchange program with the University of Jordan that will boost research and the flow of students between College Park and the Middle East country’s largest and oldest university.
The announcement of the pact by University of Maryland President Wallace D. Loh comes as a delegation led by Gov. Martin O’Malley visits Jordan on the first leg of an eight-day trip to Jordan and Israel.
back to top 

A doctor might ask for a patient’s family disease history, or exercise or smoking habits, but whether they have trouble getting food onto the table or paying energy bills is unlikely to appear on any clinic questionnaire.
Those sorts of factors could have just as much, if not more, of an impact on a person’s everyday health, argue the founders of a startup out of the Johns Hopkins University. Their company, Healthify, is giving clinics that serve largely low-income populations the means to gather and use that information.
back to top 

The Biotechnology Industry Organization (BIO) today issued the annual Scientific American Worldview Report and Bio-Innovation Scorecard. On Wednesday, April 24, the Scientific American Worldview Super Session will take place during the 2013 BIO International Convention, the global biotechnology event, in Chicago, Ill. at McCormick Place.
Moderated by Fareed Zakaria, author, journalist and host of CNN’s Fareed Zakaria GPS, the Convention Super Session is the marquee discussion forum among industry leaders on the state of biotech hubs and innovation around the globe. Panelists include Trevor Mundel, President, Global Health Program for The Bill & Melinda Gates Foundation; Robert Hariri, Chief Executive Officer, Celgene Cellular Therapeutics; Tomas Philipson, Daniel Levin Professor of Public Policy, The University of Chicago; and Sam Pitroda, Chairman, National Innovation Council, Government of India. The session will be held from 3:45pm to 5:15pm.
back to top 

May 7, 2013, 8:00am – 5:30pm (EDT)
NCI Shady Grove, 9609 Medical Center Drive, Rockville, MD 20850
This workshop will include opportunities for one-on-one meetings with representatives of many of the presenting federal organizations. Please indicate your interest and top three choices below for one-on-one meetings. NCI SBIR Program Directors will also be available to meet on the afternoon of May 6. If you are interested in setting up a meeting on May 6, please mark the appropriate selection on the registration form below.
On May 6 at 6pm (EDT), NCI SBIR will host an informal, optional meet and greet at the Cafe Deluxe in the Rio Entertainment Center (9811 Washingtonian Boulevard Gaithersburg, MD 20878 – Terrace Room). Due to government restrictions, the meet and greet will be self-pay. Please RSVP below, as space for the meet and greet is limited.
Due to space limitations, priority will be given to current or recent NCI SBIR or STTR awardees, with a maximum of two (2) representatives allowed per company. This workshop is provided free of charge to attendees (excluding food and transportation expenses).
back to top 

A Columbia University administrator is taking over a top post at Johns Hopkins University.
Robert C. Lieberman will take on the role of provost and senior vice president for academic affairs at Hopkins July 1. Lieberman is currently the interim dean of Columbia’s School of International and Public Affairs.
back to top 

Once again we’re stepping into spring with more exciting programming in health entrepreneurship. Our Rock Health Skillshare series is open to the public and provides a unique opportunity to get an in-depth look at critical issues in health innovation, as well as a peek at Rock Health HQ in Chinatown (Dim sum anyone?). Classes generally range from $25-$30 and we provide snacks, refreshments, and ample opportunity for networking afterward. Our teachers have extensive real-world domain expertise that they are enthusiastic about sharing with others.
back to top 

The Association of University Research Parks is proud to present the 18th Annual Awards of Excellence in research and science park development and practice. AURP will honor industry leaders at the 2013 International Conference in Philadelphia, Pennsylvania.
These awards bring valuable recognition to the recipients and to the industry as a whole. To nominate a park, company or an individual who has demonstrated excellence in the field, please use the below links. Each link also provides criteria and details about the award. Nominations are due by May 20, 2013.
back to top 

Today, the National Venture Capital Association (NVCA) and the Medical Innovation and Competitiveness Coalition (MedIC) released Patient Capital 3.0: Confronting the Crisis and Achieving the Promise of Venture-Backed Medical Innovation. The report, which is in its third release, highlights the contribution of venture capital to medical innovation and provides perspective on the current investment environment in the U.S. for life sciences.
“Venture-backed medical innovations still provide the critical new drugs, medical devices and diagnostics that improve and save the lives of millions of Americans, but the environment for investing in these breakthroughs has become increasingly challenging,” said Jonathan Leff, Partner at Deerfield Management and Chairman of MedIC. “While the promise of science to help solve our most pressing medical problems is extraordinary, the time, cost and uncertainty involved in building start-up companies that advance fundamental medical innovations have all risen, driving some investors away from the life sciences arena in recent years. We continue to express to policymakers the importance of promoting an environment that encourages investment in medical innovation.”
back to top 

Google Ventures has been backing startups through its venture capital fund since 2009 and offers a pretty diverse range of services to entrepreneurs. Here’s a look at the eight healthcare and life science businesses among its portfolio companies spanning DNA analysis, accelerated drug development, and oncology analytics.
23andMe The startup has helped make personal genetics and DNA analysis much more accessible to consumers. The company, co-founded by Anne Wojsicki, offers DNA test kits that for $99 and a little saliva help people better understand what conditions they are at risk for developing, whether they are carriers for any diseases that they could unwittingly give to their children. It can also provide information on whether people’s genetic makeup makes them particularly sensitive to certain drugs. It also uses results from user queries, with their consent, to conduct in-house research.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

April 30
Online Class

May 2
Baltimore Betamore

May 7- 8
Sheraton Crystal City

May 8
Morgan, Lewis & Bockius Conference Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:
 |
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
|

View Presentation
back to top 

The Montgomery County Department of Economic Development and our partner, BioHealth Innovation, Inc., invite you to visit us at Booth 1423, the Maryland Pavilion, during the BIO International Convention in Chicago, April 22 – 25.
Nearly 50 Montgomery County biotech companies, organizations, or public sector entities will attend. Twenty-six Maryland organizations, companies or public-sector entities will be represented in the Maryland Pavilion, Booth 1423, including the following from Montgomery County:
back to top 
As part of the University of Maryland’s 26th Annual Invention of the Year Awards ceremony held at the University of Maryland Golf Course on April 16, BioHealth Innovation sweetened the pot for this year’s three award winners. BHI offered each award winner strategic consulting services on federal grants or SBIR awards – valued at $2,500 per company. The BHI Commercial Relevance Program is designed to support small businesses in commercializing their technological research efforts.
The three winning inventions were:
INFORMATION SCIENCE CATEGORY:
Winner:
Time-Reversal Division Multiple Access for Wireless Broadband Communications
Feng Han, Yu-Han Yang, Beibei Wang, Yongle Wu and K. J. Ray Liu
LIFE SCIENCE CATEGORY:
Winner:
A Method for Early Diagnosis of Amyotrophic Lateral Sclerosis (ALS)
Eva Chin and Dapeng Chen
PHYSICAL SCIENCE CATEGORY:
Winner:
A Method for Rapid, Inexpensive Prototyping of Microfluidic Devices
Omid Rahmanian, Donald DeVoe
More Information
back to top 

Time-reversal techniques for optimizing broadband communication networks and rapid prototyping of microfluidics devices were among the Clark School of Engineering inventions recognized as the most promising new technologies at the University of Maryland Invention of the Year Awards.
The University of Maryland’s Office of Technology Commercialization (OTC) hosted the 26th Annual Invention of the Year Awards reception on Tuesday, April 16, 2013 from 4:30-6:00 pm at the University of Maryland Golf Course Club House.
back to top 

On the evening of April 15 as part of the InvestMaryland Challenge Award ceremony held at MICA in Baltimore, BHI presented three awards to local companies. Boss Medical LLC of Baltimore, ConverGene LLC of Gaithersburg, and Vasoptic Medical LLC of Columbia were selected for the awards based upon their high quality products under development, which have good possibility for securing federal funding with support and assistance. Each award – valued at $2,500 – consists of consulting services from BHI on Small Business Innovation Research (SBIR) or federal funding proposals. The SBIR program is designed to support small businesses in commercializing their technological research efforts.
For more information contact Ethan Byler.
back to top 

The NIH is the premier biomedical research center for the world. Its 27 Institutes and Centers employ approximately 18,000 employees doing a vast array of jobs, all supporting efforts for a healthy nation. For information on the NIH mission, goals, and Institutes and Centers, visit NIH Overview.
The Office of Translational Alliances and Coordination (OTAC) in the Division of Extramural Research Activities (DERA), National Heart, Lung, and Blood Institute (NHLBI) is seeking outstanding candidates for the Health Scientist Administrator (Business Development Specialist) position. The OTAC is charged with accelerating the translation of basic discoveries and innovations into new diagnostics, devices, and therapeutics, and facilitating the development of new technologies via Small Business Innovation Research (SBIR) initiatives. The Office facilitates identification of emerging areas of translational opportunities and provides functional integration by developing interdependent teams that leverage resources and intellect across the NHLBI, and with other NIH Institutes, agencies, and organizations. The OTAC enhances communication and coordination between existing programs, develops and coordinates strategic initiatives and Funding Opportunity Announcements (FOAs), and identifies and capitalizes on synergies to meet and enhance program goals.
View Job Posting
back to top 

Remedium Technologies Inc., a medical device company developing innovative products to control severe hemorrhaging, was awarded a $500,000 Small Business Innovation Research (SBIR) grant from the National Science Foundation to test the company’s sprayable foam for rapidly halting traumatic bleeding, company officials announce today.
In collaboration with Massachusetts General Hospital and the University of Maryland, Remedium will complete pre-clinical trials to evaluate the safety and efficacy of Hemogrip(TM) Foam in controlling non-compressible hemorrhaging, i.e., bleeding not accessible to direct pressure. Hemogrip(TM) is a high-pressure, sprayable foam that can expand into an injured body cavity, adhere to tissue and stop hemorrhaging within minutes during the expansion process. There are currently no hemostatic products available for treatment of non-compressible bleeds, which account for 85 percent of hemorrhage-related deaths.
back to top 

Two Baltimore companies took top honors in the state’s first InvestMaryland Challenge.
GrayBug, which is developing drug delivery technology; and RedOwl Analytics, a software company, were the winners of the startup business competition’s life science and information technology categories. An LED lighting company in Cecil County, i-Lighting, won the contest’s category for general industry. Some 260 startups from 26 states, including Maryland, threw their hats in the ring.
back to top 

Cleave Biosciences has added $10 million in Series A financing from new investor New Enterprise Associates, bringing its Series A total to $54 million. In the fall of 2011, Cleave raised $44 million from US Venture Partners, 5AM Ventures, Clarus Ventures, OrbiMed Advisors, Astellas Venture Management and Osage University Partners. The company is focused on cancer drug discovery and development.
Cleave is discovering novel drugs that affect protein degradation pathways. Cancer cells frequently make an excess of proteins and hence become dependent on protein degradation for their survival. By attacking key targets in these pathways, cancer cells fail to balance this excess protein synthesis with protein degradation and can no longer survive.
back to top 

The Washington region raised some $286 million in venture funding last quarter, assuming you stretch your idea of the region to include Stevensville, Md., and Blacksburg, Va. So what did we learn from three months of venture data? Here are five takeaways.
1. Deals down, dollars up: The number of deals in Q1 2013 (30 deals) was down compared with the prior quarter (36 deals), as well as Q1 2012 (45 deals), while the dollar amount was up. When 2012 went out with the crappiest quarter since 2009, the drop was attributed to the lack of big deals.
back to top 

Twelve Maryland companies, including five from the Baltimore area, received a total of $83.8 million in venture capital funding in the first quarter.
That was up 20 percent from the $69.7 million that 15 Maryland firms received in first quarter 2012, according to a MoneyTree Report by PricewaterhouseCoopers and the National Venture Capital Association. The report uses data from Thomson Reuters.
back to top 
 The Washington region raised a collective $286 million in venture capital during the first three months of 2013, thanks in large part to a $110 million financing for D.C.-based LivingSocial Inc., according to the MoneyTree report released Friday by PricewaterhouseCoopers LLP and the National Venture Capital Association. The Washington region raised a collective $286 million in venture capital during the first three months of 2013, thanks in large part to a $110 million financing for D.C.-based LivingSocial Inc., according to the MoneyTree report released Friday by PricewaterhouseCoopers LLP and the National Venture Capital Association.
At first blush, the number looks like good news for the region, a marked improvement from the $95 million raised in fourth quarter of 2012. Assuming the upswing holds for three more quarters, we just might be on track to fulfill Steve Case’s prediction of a $1 billion year.
back to top 

The purpose of the NHLBI SBIR Phase IIB Small Market Award is to provide support to Phase II SBIR awardees developing NHLBI mission-related technologies that address a rare disease or young pediatric populations. The goal of this FOA is to de-risk these technologies so that development can continue with private funding after NHLBI support ends; therefore, applicants must submit a Commercialization Plan, which should include details on any independent third-party funding that has already been secured or is anticipated during the project period. It is expected that the level of this independent third-party funding will be equal to or greater than one-third of the NHLBI funds being requested throughout the project period. Projects proposed in response to this FOA must require eventual Federal regulatory approval/clearance, and may address preclinical and/or clinical stages of technology development. Clinical trials may be proposed as appropriate, but are not required.
back to top 

Leaders of 11 top high-tech companies — including Microsoft Corp. and Intel Corp. — signed a partnership agreement Monday with a fairly new Rockville-based center on cybersecurity, pledging to work together to further that growing industry.
The companies will help the National Cybersecurity Center of Excellence — an agency formed a year ago by the federal National Institute of Standards and Technology, Montgomery County and the state of Maryland — develop leading-edge technology to combat hackers and other cyber-criminals, officials said.
back to top 
New York City nonprofit Venture for America, which provides entrepreneurship training for recent college graduates, is adding Baltimore to its roster of cities this year.
Venture was founded in 2011 to encourage entrepreneurship through practical experience. It officially launched last year in five cities: Detroit, Cincinnati, New Orleans, Las Vegas and Providence, Rhode Island. This year, Baltimore and Cleveland are on board.
back to top 

These infographics from DrugPatentWatch.com and BiologicPatentWatch.com track innovation and patent activity in the pharmaceutical and biotechnology industries:
back to top 

President Obama has challenged us all to help win the future by out-educating, out-innovating, and out-building our competitors in the 21st century.
Know someone who is doing extraordinary things to make a difference in your community? Nominate them to be a Champion of Change. We’ll consider your nominations as we feature people who are bringing about change in their communities on the White House website to share their ideas on how to win the future.
back to top 

Entrepreneurs in any industry need to start with a big idea – and a big tolerance for risk. But in health care, startups often need to take on a unique set of regulatory hurdles, complex systems and entrenched ways of getting things done to successfully build and scale.
At the TEDMED conference Thursday, a few of the industry’s most seasoned entrepreneurs and investors gave emerging startups a dose of advice. Here are a few of their tips:
back to top 

U.S. Small Business Administration chief Karen Mills was in Baltimore on Thursday to highlight the agency’s education program for entrepreneurs.
Baltimore was one of the first cities to launch SBA’s Emerging Leaders program, formerly called E200 back in 2008. The program offers a seven-month course in which a select group of business owners learn the skills they need to grow their companies.
back to top 
There’s been discussion recently about a lack of life science venture capital enthusiasm for emerging digital health companies. I happen to feel that this concern is misplaced. I engaged industry and opinion leaders on this subject plus have my own thoughts on the digital health funding environment.
In his latest article in Forbes, contributor David Shaywitz characterizes life science venture capitalists as just “kicking tires” on potential digital health investments, citing fears from prominent investor Nimesh Shah that innovations in digital health are “merely a bubble,” and that these firms lack a “real biz model.”
back to top 

The Universities at Shady Grove (USG) and the Rockville Institute come together annually to offer this dynamic, interactive seminar series. The series stimulates an exchange of ideas among practitioners, researchers, policymakers, students, and the public at large about contemporary social issues. Seminars are free and open to the public.
LGBT Youth in Foster Care: Challenges and Strategies
Thursday, March 14, 2013 – 4:30pm
What Is a Meaningful Use of an Electronic Health Record?
Tuesday, April 2, 2013 – 4:30pm
Enhancing Learning in STEM Through the Creation of Master Teachers
Wednesday, April 24, 2013 – 4:30pm
back to top 

The U.S. market for remote patient monitoring, valued at $104.5 million in 2012, is forecasted to reach $296.5 million by 2019, a compound annual growth rate of 16 percent, according to a new report from GBI Research.
Chronic diseases represent an overwhelming burden for healthcare systems in developed countries, due to increasingly elderly populations, and a clear need exists for remote patient monitoring to help lift the load off stretched healthcare resources, according to the report Remote Patient Monitoring Market to 2019 – Potential to Reduce Healthcare Cost Burden and Improve Quality of Care to Drive Future Growth.
back to top 

With nearly half a billion dollars raised in venture capital funding for health information technology, the first three months of 2013 represented a “record quarter,” according to Mercom Capital Group.
Some $493 million was raised industry-wide, according to Mercom’s 2013 Healthcare IT Funding and M&A Report, in twice as many deals as the previous quarter (104, up from 51). There were nearly four times as many early stage deals – 42, up from 14 – compared to the fourth quarter of 2012.
back to top 

The University of Maryland, Baltimore County and Northrop Grumman Corp. last month expanded their Cync cybersecurity program with three new companies, including the program’s first international one. The three firms joined the five companies currently at bwtech@UMBC Research and Technology Park in Catonsville.
back to top 

Eight years of work, thousands of researchers around the world, $1 billion spent — and finally it was done. On April 14, 2003, a decade ago this week, scientists announced that they had completed the Human Genome Project, compiling a list of the three billion letters of genetic code that make up what they considered to be a sort of everyperson’s DNA.
back to top 

The need for innovation in healthcare has arguably never been greater. A range of factors, from aging world populations to rising standards of living in developing countries, are poised to drive long-run demand for innovative drugs, devices and medical technologies that can improve outcomes and reduce costs.
Ironically, however, funding for healthcare innovation remains in short supply. As industry participants are keenly aware, life science venture capital financing – which has played a critical role in helping translate research ideas into commercially useful medical technologies – is becoming increasingly scarce.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

April 22
Sheraton Crystal City

April 23-24
Sheraton Crystal City

April 23-25
The Westin Westminster

April 23
Bethesda Blues and Jazz Supper Club

April 24
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

On the evening of April 15 as part of the InvestMaryland Challenge Award ceremony held at MICA in Baltimore, BHI presented three awards to local companies. Boss Medical LLC of Baltimore, ConverGene LLC of Gaithersburg, and Vasoptic Medical LLC of Columbia were selected for the awards based upon their high quality products under development, which have good possibility for securing federal funding with support and assistance. Each award – valued at $2,500 – consists of consulting services from BHI on Small Business Innovation Research (SBIR) or federal funding proposals. The SBIR program is designed to support small businesses in commercializing their technological research efforts.
For more information contact Ethan Byler.
back to top 

Richard Bendis will speak at the Translational Research Forum during the 2013 BIO International Convention in Chicago. During the forum, speakers will address issues with current translational research models, explore new funding and collaboration opportunities, evaluate how to apply them to federal, academic, and private institutions. The session will take place Monday, April 22, from 4:35 p.m. – 5:25 p.m.
More Information
back to top 

Date: Wednesday, May 8th, 2013 9:30am-5:00pm
Location: Morgan, Lewis & Bockius Conference Center, 1111 Pennsylvania Ave., NW Washington, DC 20004-2541
National Brain Tumor Society is bringing together brain tumor researchers and industry executives to discuss how to move research from bench to bedside.
- Biophrama executives and VC investors will share their criteria for investment and licensing of academic research.
- Panelists include:
- Lauren Abrey
Global Development Team Lead, Genetech/Roche
- Neil Exter
Partner, Third Rock
- Brian Gallagher
Partner, SROne
- Michael Glutch
Managing Director, Medimmune Ventures
- Perry Nisen
Senior VP of Science Transactions, Pfizer
- Nation Brain Tumor Society-funded researchers will present brain tumor drug candidates ready for early stage investment and/or licensing.
back to top 
Friday, May 24 • 12:00 – 2:00 pm • Marriott Bethesda North Conference Center

Join the Montgomery County Department of Economic Development to celebrate small businesses. Attend the inaugural Small Business Awards program.
The award luncheon will provide countless opportunities to show your appreciation for the estimated 33,000 small businesses of Montgomery County—businesses that contribute directly to the strength of the area’s economy. Eight awards will be presented to eight distinct companies.
back to top 

Cleave Biosciences has added $10 million in Series A financing from new investor New Enterprise Associates, bringing its Series A total to $54 million. In the fall of 2011, Cleave raised $44 million from US Venture Partners, 5AM Ventures, Clarus Ventures, OrbiMed Advisors, Astellas Venture Management and Osage University Partners. The company is focused on cancer drug discovery and development.
Cleave is discovering novel drugs that affect protein degradation pathways. Cancer cells frequently make an excess of proteins and hence become dependent on protein degradation for their survival. By attacking key targets in these pathways, cancer cells fail to balance this excess protein synthesis with protein degradation and can no longer survive.
back to top 

When he was young, Daniel J. Abdun-Nabi wasn’t all that interested in subjects like biology. He gravitated toward political science and the law in college, and worked for the federal Securities and Exchange Commission soon after graduating.
“I went out of my office and saw the Capitol. It was awe-inspiring,” Abdun-Nabi, 58, who grew up near Boston, said of working in Washington, D.C.
back to top 

David Mott at New Enterprise Associates has spearheaded the development of an orphan drug accelerator that plans to hunt down the best in vivo-stage assets in academia, biopharma and the nonprofits, assemble them in a portfolio of rare disease therapies and mold them into pipeline programs that can be spun out into a slate of new biotech companies.
NEA’s high-profile biotech partner Mott–who helmed MedImmune until AstraZeneca ($AZN) came along and scooped it up for $15.6 billion–partnered with Pfizer Ventures and gained the support of Alexandria Real Estate Equities in piecing together the initial $16 million in startup funds for the newly coined Cambridge, MA-based Cydan.
back to top 

GlaxoSmithKline, Britain’s biggest drugmaker, is placing a small but important bet on a new way of treating diseases by targeting electrical signals in the body.
The company said on Wednesday it would offer a $1 million prize to stimulate innovation in the field, as well as funding up to 40 researchers working in external laboratories.
back to top 

The Global Virus Network plans to move its headquarters to the University of Maryland BioPark in Baltimore, officials announced Thursday.
Emerging pandemic viral threats and current viral killers are a worldwide problem. For this reason, the nonprofit GVN brings together the top medical virologists from more than 30 institutions in 21 countries, to promote international collaborative research, education and advocacy.
back to top 

Pluristem Therapeutics (NASDSQ: PSTI) announced today that following favorable preclinical studies, United Therapeutics Corporation received approval to perform a human Phase I study in Australia using Pluristem’s PLacental eXpanded (PLX-PAD) cells in patients diagnosed with Pulmonary Arterial Hypertension (PAH). On June 20, 2011 United Therapeutics and Pluristem entered into a licensing agreement pursuant to which United Therapeutics will develop, market and sell Pluristem’s PLX-PAD cells for PAH.
PAH is characterized by abnormally high blood pressure in the arteries of the lungs and leads to an increased workload on the right side of the heart.
back to top 

Pieris AG and The Sanofi Group have jointly agreed to expand their ongoing discovery and development partnership to include a novel multispecific Anticalin® program. Under the existing framework of the 2010 agreement, the new program will entitle Pieris to an upfront payment from Sanofi and committed research funding as well as payments for the achievement of research, preclinical, regulatory and commercial milestones. Specific financial terms were not disclosed.
back to top 

A test that detects human papillomavirus, a virus directly related to cervical cancer, has hit the market following approval by the China Food and Drug Administration.
“The careHPV test is faster, better and cheaper,” says Qiao Youlin, a respected epidemiologist, who headed a research project into the development of careHPV between 2003 and 2008. The research was assisted by the Bill & Melinda Gates Foundation, which contributed $13 million to the project.
back to top 

Company: MedImmune Booth/Stand: 3344 Event: 2013 BIO International ConventionApr 22 – 25 2013 Chicago IL US
About MedImmune
MedImmune is the worldwide biologics research and development arm of AstraZeneca a global innovation-driven biopharmaceutical business that focuses on the discovery development and commercialization of small molecule and biologic prescription medicines. MedImmune is pioneering innovative research and exploring novel pathways across key therapeutic areas including respiratory inflammation and autoimmunity; cardiovascular and metabolic disease; oncology; neuroscience; and infection and vaccines. MedImmune has approximately 2500 employees globally with its headquarters located in Gaithersburg Md. one of AstraZeneca’s three global R&D centers. For more information please visit www.medimmune.com.
back to top 

When engineers at this university learned of a Baltimore neurosurgeon’s struggle to operate on sensitive parts of the brain, they designed a tiny robot to maneuver around more precisely than instruments held by a human hand during surgery — something Loh said never would have happened if the neurosurgeon and robotic engineers had not met. Now, one year into the partnership, the universities have launched a collaborative public health school, developed a joint biomedical informatics and bioimaging center and created a research and innovation program.
“When you have a formal structure for a partnership and encourage people to talk to each other, this is what the partnership between the universities is all about,” Loh said. “They have the problems; we have the solutions.”
back to top 

The Maryland Technology Development Corp. has named a Stifel Nicolaus Weisel executive to manager the organization’s four venture capital funds.
Christopher College was hired as managing director for the recently established Tedco Capital Partners.
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association with more than 400 biotechnology and technology members employing more than 200,000 in the region, announced the finalists for its 25th Annual Dinner and Awards Celebration. Winners will be announced at the awards dinner on May 16 at the Bethesda North Marriott Hotel and Conference Center.
“This year’s finalists are a testament to the ingenuity and vibrancy of Maryland’s technology and life sciences community,” said Larry Letow, TCM’s chairman. “Their innovations advance the cause of a healthier, safer world while fostering job creation and prosperity here in Maryland. Simply put, Maryland’s tech and biotech companies are essential to our long-term economic growth. The TCM’s Awards Celebration is a great way to celebrate our members’ tremendous achievements to date and the bright future that lies ahead.”
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association with more than 400 biotechnology and technology members employing more than 200,000 in the region, today commended state policy makers for advancing key priorities for the tech community during this year’s legislative session. The 2013 session concluded on Tuesday.
“Maryland’s policy makers delivered on our biggest priorities: stronger incentives for R&D and biotechnology, new incentives for cybersecurity investments, and corporate tax code certainty for employers,” said Larry Letow, TCM’s chairman. “We have many strong advocates in Annapolis who understand that Maryland’s technology and biotechnology companies are catalysts for innovation, job growth and prosperity.”
back to top 

One of the world’s top physicians, Dr. Eric Topol, has a prescription that could improve your family’s health and make medical care cheaper. The cardiologist claims that the key is the smartphone. Topol has become the foremost expert in the exploding field of wireless medicine. Dr. Nancy Snyderman reports.
back to top 

I launched a literary magazine in college. I actually thought that was a good idea. After a few too many 16-hour days of hustling ads and desperately scrounging for content, my grades tanked and my blood pressure skyrocketed. I learned that special kind of panic that comes from trying to execute on the inflated ambitions of a 20-year-old. The process consumed everything. (For the record, I regret nothing.)
My point is: The university setting is great for being entrepreneurial. But it takes organization and drive to hold it all together — something I realized I didn’t have. At least some of these young founders do.
back to top 

Nominate your best startups to present to a select group of invitation-only investors and Global 1000 companies as part of the Virtual Startups Showcase (VSS) and the Exits Startups Showcase + Conference in San Francisco
NOMINATION DEADLINES
- For University Startups: Friday, April 26, 2013
- For Accelerator, Angel Investor and VC Portfolio Companies: Friday, June 14, 2013
The National Council on Entrepreneurial Tech Transfer (NCET2) asks universities, accelerators, Angel investors and VC’s to nominate their best startups and have them present to a select group of invitation-only investors and Global 1000 companies.
back to top 

The need for innovation in healthcare has arguably never been greater. A range of factors, from aging world populations to rising standards of living in developing countries, are poised to drive long-run demand for innovative drugs, devices and medical technologies that can improve outcomes and reduce costs.
Ironically, however, funding for healthcare innovation remains in short supply. As industry participants are keenly aware, life science venture capital financing – which has played a critical role in helping translate research ideas into commercially useful medical technologies – is becoming increasingly scarce.
back to top 

HHS Secretary Kathleen Sebelius joins Ellen Murray, the Assistant Secretary for Financial Resources (ASFR), and HHS agency leaders to announce the President’s HHS budget for Fiscal Year 2014.
back to top 

The University of Maryland (UM) BioPark announced today that the Global Virus Network (GVN) is the Park’s newest tenant. The GVN is a non-profit organization comprised of the top medical virologist in more than 30 institutions spanning 21 nations – and growing. GVN’s mission is to combat emerging pandemic viral threats and current viral killers through international collaborative research, training the next generation of medical virologist, education and advocacy.
Said Jim Hughes, President, Research Park Corporation, University of Maryland Baltimore, “It’s exciting for the UM BioPark to include the prestigious Global Virus Network as one of our tenants. We are also pleased to be able to offer GVN continued close proximity to its Scientific Director, Co-Founder, and world-renowned virus researcher Dr. Robert Gallo from the UM School of Medicine, whose Institute of Human Virology is located conveniently just down the street from the GVN’s new BioPark headquarters.”
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

April 14-16
Washington, DC Metro Area

April 15
MICA’s Brown Center

April 16
Legg Mason Tower

April 17
UMBC Campus

April 22
Sheraton Crystal City
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Pictured: Rich Bendis, BHI; Arkesh Mehta, Chikujee Therapeutics; Stephen Yoder, Pieras AG; Rick Soni, Rexahn Pharmaceuticals
Growlers was “abuzz” on March 20 with a large and diverse gathering of biotech professionals attending the monthly BioBuzz networking event. BHI CEO Rich Bendis welcomed the crowd and talked about the latest happenings at BHI. According to Andrew Eckert, “We received a lot of positive feedback on the location and found a number of folks were interested in speaking to somebody from BHI. I’m pretty confident this was one of the largest, if not THE largest turnout ever for a BioBuzz event.”
BioBuzz is working to build a stronger community by bringing the biotech workforce out of the labs to culture relationships, instead of just cells.
back to top 

On April 2, BHI invited a team of NIH Office of Technology Transfer interns working with BHI EIR Todd Chappell for a Social Meeting. Also in attendance were two guest speakers. Bart Kus, Senior Manager of Portfolio Management MedImmune, spoke about his career track from the lab into business development. Errol Levy, EU Diplomat (Research and Innovation Counsellor) for the European Commission talked about opportunities in Europe and the upcoming Destination Europe Conference on April 11 in DC. It was a great event for all who attended.
back to top 

Maryland has invested $500,000 in Cytomedix Inc., a regenerative medicine company in Gaithersburg.
The investment was made through the state’s Maryland Venture Fund and InvestMaryland program. The state said the money is part of a $27.5 million fundraising round by Cytomedix (OTC: CMXI) that will result in the company adding 13 new employees to its current staff of 55.
back to top 

Tom Sadowski, President and CEO, of the Economic Alliance of Greater Baltimore has been appointed to UMBC’s Public Policy External Advisory Board. Created in 2003, the External Advisory Board provides advice and guidance to UMBC’s Department of Public Policy and the Maryland Institute for Policy Analysis and Research (MIPAR). An important part of their mission is to bring the University’s public policy expertise to bear on the concerns of the communities they serve.
back to top 

GlaxoSmithKline is trying to expand the market for its Benlysta drug beyond lupus by launching a major study of the medicine as a treatment for a serious blood vessel disorder.
GSK acquired full control of Benlysta when it bought Human Genome Sciences for $3 billion last year. Current sales of the drug are modest, at 70 million pounds ($106 million) in 2012, but GSK hopes it will become a major seller.
back to top 

BioMed Realty Adds 1.6 Million Square Foot Portfolio & Expands University / Research Institution Segment to 18% of Annualized Base Rents
BioMed Realty Trust, Inc. (NYSE: BMR) announced today that it has entered into a definitive agreement to merge with Wexford Science & Technology, LLC, a subsidiary of Wexford Equities, LLC, furthering BioMed Realty’s position as the leading provider of real estate to the life science industry. Wexford Science & Technology is a private real estate investment and development company that owns and develops institutional quality life science real estate for academic and medical research organizations, and that boasts well-regarded skills for urban development and redevelopment of life science real estate. The aggregate consideration for Wexford Science & Technology is approximately $640 million, excluding transaction costs and subject to adjustment based on working capital levels and construction and development costs incurred prior to closing. Wexford Science & Technology will operate as a wholly owned subsidiary of BioMed Realty.
back to top 

And then there were nine.
The Maryland Department of Business and Economic Development has named three finalists in three separate categories of the final round of the InvestMaryland Challenge. The winning companies, to be announced April 15, will receive $100,000 in top prizes and $125,000 in cash and in-kind awards as part of the Maryland Venture Fund’s first business competition.
InvestMaryland is a state venture capital initiative managed by DBED and the state-run Maryland Venture Fund. The initiative raised $84 million through a tax credit auction in 2012 to invest in startup companies.
back to top 

AstraZeneca ‘s ( AZN ) worldwide biologics research and development (R&D) unit, MedImmune recently acquired AlphaCore Pharma, a biotechnology company based in Michigan. Financial terms of the deal were not disclosed by the companies.
We note that the acquisition of AlphaCore Pharma adds ACP-501 (a recombinant human lecithin-cholesterol acyltransferase/ LCAT enzyme) to AstraZeneca’s pipeline. The candidate is being developed for acute coronary syndromes (ACS) and other high-risk atherosclerosis conditions for the rapid removal of tissue cholesterol.
back to top 

Johns Hopkins researcher Se-Jin Lee was named Wednesday as the 2013 recipient of the Ho-Am Prize in Medicine, which recognizes outstanding accomplishments in medical research that pave the way to conquering disease. It is awarded each year to an ethnic Korean and is sometimes referred to as “Korea’s Nobel.”
Lee is best known for his discovery of myostatin, a hormone that regulates muscle mass, and related work that lays the foundation for new therapies for conditions such as muscle wasting and muscular dystrophy.
back to top 

Those who join MedImmune feel a sense of ownership about their future. They thrive with a recognized leader in the biotechnology industry and the wholly-owned subsidiary of AstraZeneca plc.
Here, you will join passionate professionals who advance science, technology and medicine to develop products designed to help people live better lives. You will excel in an environment characterized by respect, integrity and growth opportunities…that encourages both individual contribution and collaborative entrepreneurial thinking. Our products and/or product candidates are designed to address areas of need in infection, oncology, respiratory disease and inflammation, cardiovascular/gastrointestinal disease and neuroscience. Explore a MedImmune career as we strive to better more lives, more often, around the world.
back to top 

The Maryland House of Delegates has passed a measure to adjust how money from a state venture capital fund can be used to try to make it more effective.
The House voted 107-25 on Saturday for the bill. That sends it to the Senate to consider amendments delegates made to the measure.
back to top 

WIScience Scholarship: The Premier Catalyst for Developing Women Leaders in Science
Are you nearing completion of your graduate degree or fellowship, and wondering how you’re going to transition into the healthcare industry? Do you get nervous thinking about how to identify the career in healthcare that fits you best? Do you wish you had a mentor to discuss these concerns with? If so, then you are the perfect candidate to apply for the WIScience Scholarship: The Premier Catalyst for Developing Women Leaders in Science offered by the Mid-Atlantic chapter of the Healthcare Businesswomen’s Association (HBA).
back to top 
President Obama unveils a bold new research initiative designed to revolutionize our understanding of the human brain, and discusses the importance of investing in American innovation to create jobs and strengthen our economy. April 2, 2013.
back to top 

Since the passage of the Jumpstart Our Business Startups (JOBS) Act (H.R. 3606) last year, the financial and medical press has been buzzing about the potential for crowdfunding to revolutionize fundraising for early-stage biotech companies. Even more recently, a portal that is exclusively dedicated to crowdfunding biotech and healthcare start-ups launched at medstartr.com. Although there may be reason for biotech entrepreneurs to be excited about crowdfunding, there are significant limitations and risks to this approach as well.
back to top 

There’s an interesting trend I’ve noticed with at least one healthcare accelerator I have been tracking. The members of the startups for the most part have increasingly more industry experience and seem more adept at finding pain points and delivering a workable solution to members of the healthcare ecosystem. And if the successes of Blueprint Health’s latest graduating class are anything to go by, healthcare companies are getting increasingly receptive to working with startups that can help them address some of the demands of the Affordable Care Act and some of its impacts with more innovative solutions.
back to top 

Seeking to maximize ongoing research, innovative ideas and revenue-generating opportunities, more schools are creating entrepreneur-in-residence programs — and letting the experts do the work.
With millions of dollars of research funding and teams of experts at their disposal, more universities are seeking ways to turn research breakthroughs into business opportunities.
back to top 

DreamIt Health, a new Philadelphia-based, health-focused chapter of incubator DreamIt Ventures, has announced its first class of ten startups. Independence Blue Cross (IBC) and Penn Medicine are sponsoring the class and Venturef0rth is providing the working space for the companies. The new accelerator was launched in December 2012.
The startups will be provided with up to $50,000 in funding, office space, mentoring, and resources for developing and testing health-related products. The incubator will last four months, and companies will receive coaching from both entrepreneurs and health care executives. DreamIt Ventures has launched 80 companies over the past four years, including one health-related startup, 1DocWay. Supporting sponsors include global professional services company Towers Watson, and law firms Morgan Lewis, and Pepper Hamilton.
back to top 

Among 400 applications from 22 countries, 13 early stage healthcare companies have been chosen for a three-year entrepreneurship class run by StartUp Health and GE, according to a statement from the companies. The class members will each be assigned a GE mentor who matches their business model and get access to the resources the Fortune 50 company can wield to help scale their consumer health innovations. That could include working with GE Healthymagination, GE Healthcare, GE Capital, or some of its business units, depending on the members’ specific needs.
back to top 

Digital health incubator Rock Health released data today that shows an increase in funding for med-tech startups in the first quarter of 2013.
Thirty-seven health deals were valued at a total of $365 million, which is 35 percent higher than the first quarter of 2012, “suggesting that 2013 will be another record year for the digital health industry,” the blog post reads.
back to top 

Philadelphia has reached a milestone in its efforts to be recognized as a healthcare and life sciences hub with the start of its first health IT accelerator.
DreamIt Health’s inaugural class includes 10 startups. The four-month program co-sponsored by Independence Blue Cross and Penn Medicine includes members seeking to develop tools for healthcare providers to speed up diagnoses and improve outcomes.
back to top 

GlaxoSmithKline ( GSK ) recently announced that it has initiated a phase III study to evaluate the use of Benlysta (belimumab) in patients suffering from anti-neutrophil cytoplasmic antibodies (ANCA) positive vasculitis.
The multi-centre, randomized, double-blind phase III study will assess the efficacy and safety profile of Benlysta in combination with azathioprine as a maintenance therapy in ANCA positive vasculitis patients.
back to top 

A big-data revolution is under way in health care. Start with the vastly increased supply of information. Over the last decade, pharmaceutical companies have been aggregating years of research and development data into medical databases, while payors and providers have digitized their patient records. Meanwhile, the US federal government and other public stakeholders have been opening their vast stores of health-care knowledge, including data from clinical trials and information on patients covered under public insurance programs. In parallel, recent technical advances have made it easier to collect and analyze information from multiple sources—a major benefit in health care, since data for a single patient may come from various payors, hospitals, laboratories, and physician offices.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
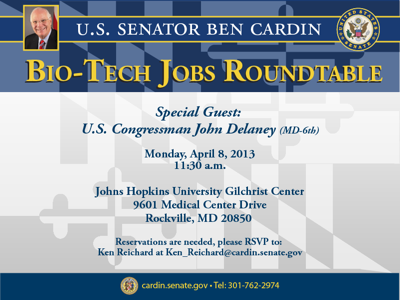
April 8
Johns Hopkins University, Gilchrist Center

April 9
Johns Hopkins Hospital

April 11
Webinar

April 14-16
Washington, DC Metro Area

April 15
MICA’s Brown Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
– Multiple SBIR/STTR Submission Deadlines Quickly Approaching –
 BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today the launch of its Commercial Relevance Program (CRP). BHI’s CRP is designed to help life science companies navigate the complicated process of preparing applications for federal funding, inclusive of Small Business Innovation Research (SBIR), Small Business Technology Transfer (STTR), and other federal government awards. BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today the launch of its Commercial Relevance Program (CRP). BHI’s CRP is designed to help life science companies navigate the complicated process of preparing applications for federal funding, inclusive of Small Business Innovation Research (SBIR), Small Business Technology Transfer (STTR), and other federal government awards.
“The federal grant application process can be very complex. Based on 2012 data released by the National Institutes of Health (NIH), Maryland ranks 32nd out of 50 states with regard to SBIR award success rates. We would like to improve on this – and are confident that the CRP will help companies be more successful with their submissions,” said Ethan Byler, Director, Innovation Programs, BioHealth Innovation, Inc.
“At the end of the day, it’s about helping Central Maryland companies to get the best results possible as they seek out federal grant awards as a means of non-dilutive funding,” he added.
The CRP incorporates a pre-proposal review by knowledgeable BHI staff and advisors prior to a life science company’s submission of a full proposal. Through this review process, applicants will receive a set of recommendations and tips for troubleshooting their proposal for federal funding.
Interested life science companies in Central Maryland should contact BHI today for more information. The following federal agency SBIR/STTR deadlines are fast approaching:
About BioHealth Innovation, Inc.
BioHealth Innovation, Inc., is a regional innovation intermediary focused on commercializing market-relevant bio-health innovations and increasing access to early-stage funding in Maryland. Learn more at www.biohealthinnovation.org.
back to top 

back to top 

Congratulations to Elaine Amir! Elaine, executive director of the Montgomery County Campus of Johns Hopkins University, was named to The Daily Record’s 2013 list of Maryland’s Top 100 Women.
The announcement brought kudos from JHU interim provost Jonathan Bagger.
back to top 

BioMed Realty Adds 1.6 Million Square Foot Portfolio & Expands University / Research Institution Segment to 18% of Annualized Base Rents
BioMed Realty Trust, Inc. (NYSE: BMR) announced today that it has entered into a definitive agreement to merge with Wexford Science & Technology, LLC, a subsidiary of Wexford Equities, LLC, furthering BioMed Realty’s position as the leading provider of real estate to the life science industry. Wexford Science & Technology is a private real estate investment and development company that owns and develops institutional quality life science real estate for academic and medical research organizations, and that boasts well-regarded skills for urban development and redevelopment of life science real estate. The aggregate consideration for Wexford Science & Technology is approximately $640 million, excluding transaction costs and subject to adjustment based on working capital levels and construction and development costs incurred prior to closing. Wexford Science & Technology will operate as a wholly owned subsidiary of BioMed Realty.
back to top 

The Maryland Venture Fund has awarded $300,000 to Adlyfe, a Rockville diagnostic test developer, as part of the state’s InvestMaryland program.
Adlyfe, which is developing an early detection test for Alzheimer’s disease, is also receiving a $200,000 grant from the Maryland Biotechnology Center.
back to top 

Emergent BioSolutions, Inc., a global specialty pharmaceutical company, is generating fresh ideas from Michigan State University students at its Lansing location to improve operations.
Emergent BioSolutions, the Roscommon-based Lear Corp., the Saginaw-based Mistequay Group, Ltd. and approximately five other companies hosted more than 20 undergraduate and graduate business and engineering students on various projects. The initiative is the brainchild of Bill Demmer, the CEO of the Lansing-based Demmer Corp., who donated $5 million to the university to create the Marnie Demmer Center for Business Transformation in late 2011, MLive.com reports.
back to top 

Sure, New Enterprise Associates is much, much bigger than most venture capital firms. And not all of their deals fall neatly into what we think of as traditional Series A-B-C venture.
NEA invests in private hospital systems in China. It does the occasional PIPE deal. It does big-ticket “growth” fundings that look a whole hell of a lot like private equity.
But NEA is definitely not a private equity firm, as it is here described in The Wall Street Journal.
back to top 

Two announcements on Monday and Thursday of last week signaled a fundamental strategic and physical shift for biopharma giant AstraZeneca, including the establishment of a new corporate headquarters in a new UK location; the shift of small molecule and biologics R&D to three strategic centers around the world; and an eventual overall headcount reduction of 5,050 between now and the end of 2016.
On Monday, March 18, the company announced its intent to focus the R&D in three places, and to fully implement these plans by 2016:
back to top 

As the CEO of Pulse8, John Criswell works with health insurance exchanges and long-term care facilities on big data and analytics, which gives him a purview into the back-end integration required for states and the federal government to setup HIXs. Yet, he’s optimistic that the gvoernment will meet the October 1, 2013 deadline.
Government Health IT Editor Tom Sullivan spoke with Criswell about the complexity of standing up HIXs, how the exchanges might fare come October, and the overarching opportunities that both HIX and health information exchanges will create as they amass mammoth data sets.
back to top 

The following eight companies have joined our campus community within the past few months. Welcome! We look forward to hearing more about your work.
back to top 

Monday, April 15th 5:30 – 8:30 p.m. at MICA’s Brown Center
After months of submitting business plans, scoring applications, developing special awards and partnering with us, it’s time to celebrate entrepreneurship! Please join us April 15th at Maryland Institute College of Art’s Brown Center as we announce the top three $100K winners.
back to top 
Tech entrepreneurs will soon find a new home at Innovation Crossroads, the Eastern Shore’s first, full-service business incubator.
Gov. Martin O’Malley and the department of public works recently approved $1.2 million in funding from the Maryland Department of Business and Economic Development for construction of the incubator, the Dorchester County Council announced on Tuesday.
Innovation Crossroads is the planned anchor tenant of the newly constructed Cambridge-based Regional Technology Park.
back to top 

“Transformation” is the best description of what is happening in health care right now. We are seeing historic changes in how health care is administered in the United States—with increased focus on quality of care versus just paying for a service. We are seeing changes in how people can enroll in health insurance—with the upcoming establishment of a new market place that will help more people get insured in this country than ever before. And, we are seeing changes in how people understand and make decisions about their own health—with an increasing number of tools and services becoming available to help individuals access health information and manage their own personal health data.
back to top 

The Congressional hearings debating the regulatory framework for mobile health devices and apps made me think about how we view mobile health apps and the criteria we use to determine what makes some better than others.
And as we all think in terms of the NCAA college basketball tournament around this time, I thought we would do a mini tournament using mobile health apps.
back to top 

As we come to the close of Q1 of 2013, LSN has compiled some insight into three of the hottest subsectors being targeted by investors in the medical technology space. New technologies are surfacing that are changing treatment philosophies, and more importantly, medtech is attracting a whole new class of investors such as corporate venture from the consumer electronics, digital media, and telecom space. Here are the three trend areas that are changing the world from the medtech investor perspective:
back to top 

This the title [paraphrased] of a keynote given last week by Jonathon Bush, the CEO of AthenaHealth, at the Xconomy Mobile Madness conference in Cambridge, Ma. What follows are my notes from the speech. The ideas are Jonathon’s. I really like what he’s saying and mostly agree, and he puts it very well.
AthenaHealth’s base business is medical practice billing in the cloud. This is an example of technology-enabled transformation of the health care business system, which we believe will be a big opportunity for entrepreneurs going forward. Founded in 1997, AthenaHealth is now a successful public company (ATHN).*
back to top 

A week after the Congressional hearings discussed how the US Food and Drug Administration’s impending regulatory guidelines (expected by October) will impact mobile health apps, aspiring and current digital health entrepreneurs may be rethinking their path. In a report dubbed “FDA 101,” health startup accelerator Rock Health has compiled a handy report to help digital health entrepreneurs assess whether their mobile health apps would be classified as medical devices by the FDA and require 510(k) clearance.
Some of the pressure to clear the waters has come from technology developments that have simply outpaced the regulatory environment. Another is that provisions of health care reform call for making greater use ofremote monitoring, to reduce healthcare costs. Providers are pretty keen to reduce costs where they can as well. Remote monitoring would be particularly helpful when it comes to patients with chronic conditions, such as congestive heart failure or diabetes or both, so they can have shorter hospital stays and care managers can be alerted for any changes in their vital signs.
back to top 

President Barack Obama’s top healthcare adviser acknowledged on Tuesday that costs could rise in the individual health insurance market, particularly for men and younger people, because of the landmark 2010 healthcare restructuring due to take effect next year.
U.S. Health and Human Services Secretary Kathleen Sebelius said definitive data on costs will not be available until later this year when private health plans become authorized to sell federally subsidized coverage on new state-based online marketplaces, known as exchanges.
back to top 

Many of the changes in the healthcare industry outlined by the Affordable Care Act and HITECH Act call for shifting to healthcare IT for helping physician practices run more efficiently to helping guide clinical outcomes. But the shift from paper based to digital records isn’t just happening among providers. It is carrying over into other industries that healthcare intersects such as pharmaceuticals, medical devices and more robust collaborations with payers.
Andrew Khouri of Quaker Partners, who also mentors Blueprint Health accelerator companies, highlighted some needs in healthcare that are creating interesting investment opportunities and some investment trends at a recent event at the University City Science Center. Khouri acknowledged the “industry is in turmoil” because there’s uncertainty about how the changes will work in practice from the transformation of payment models to setting up health insurance exchanges. But there’s so much momentum to reduce costs and improve outcomes that there is a lot of opportunity.
back to top 

The Series A crunch has left the realm of Bigfoot and Nessie and is entering the realm of truth, at least according to Fenwick & West.
The law firm released the results of its 2012 Seed Financing Survey today, which found that the number of startups obtaining Series A financing after a seed round declined significantly in 2012.
back to top 

As expected, the third and final (for now, at least) congressional hearing focused on the FDA’s regulation of mobile medical apps was the only one that provided new information for those already well-versed in mobile health. While the past two days of testimonies likely helped members of Congress better understand the concerns of those working or lobbying for the healthcare and technology industries, those testimonies did not reveal anything new about what was actually going on at the FDA.
This morning the FDA’s Christy Foreman, who serves as the Director of the Office of Device Evaluation at the Center for Devices and Radiological Health, shared a number of insights about mobile medical app regulation that before today had not been revealed. Here are five things we learned from the FDA’s testimony during the congressional hearing today:
back to top 

Why not explore what’s to gain from crowdfunding when there’s nothing to lose? MedStartr’s co-founder and CEO Alex Fair explains.
Alex Fair, Co-Founder & CEO of MedStartr Thinking about joining the crowd? We know, your mother probably told you not to get mixed up with the wrong kind. But according to MedStartr’s Alex Fair, there’s a lot right with the mob mentality growing among today’s entrepreneurs; crowd communities are creating equitable springboards for promising startups. That’s right, we’re talking about crowdfunding. After all, there is strength in numbers— especially from those that come with dollar signs.
back to top 

No action is good action. At least for online investment platform the Funders Club that today received a ‘no-action letter’ from the SEC stating that it will not recommend enforcement action.
The letter is a green light for the FundersClub and validation that its investment model is legal. The news is significant for the venture capital and finance industries, as well as startups looking for more flexible methods of fundraising.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

April 3
Online Webinar

April 5
Riggs Alumni Center

April 8
Johns Hopkins University, Gilchrist Center

April 9
Johns Hopkins Hospital

April 11
Webinar
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
| | | | | | |