Washington, DC, July 10 2025 – Nanochon, a Washington, DC and Baltimore, MD-based orthopedic device biotech company developing an implant for treating articular cartilage defects in the knee, is thrilled to announce that it was granted Health Canada Approval for their first in human clinical trial.
The trial will be led by Principal Investigator, Dr. Fathi Abuzgaya, who will collaborate with Sports Medicine Specialists, Drs. Joel Lobo, Kajeandra Ravichandiran and Marcin Kowalczuk as Sub-Investigators, at the Durham Bone & Joint Specialists (DBJS), located in Ontario, Canada. Dr. Fathi Abuzgaya, is an orthopedic surgeon by training and an esteemed researcher with a track record over the last 25 years, leading +600 clinical trials in all phases and therapeutic areas.
“Our team is delighted that we are the first center to start enrolling participants for the Chondrograft™ study, which represents a novel, minimally invasive treatment for patients with loss of or damaged knee cartilage,” Dr. Abuzgaya commented. “DBJS is a multidisciplinary musculoskeletal center providing non-operative and operative treatments with accessibility to a variety of sub-specialties. Our study site with dedicated researchers enables greater, more efficient patient recruitment for clinical research and we are privileged to support Nanochon.”
The Nanochon Chondrograft™ System is being evaluated in a prospective, 10 patient, early feasibility study for use in knee cartilage restoration in eligible patients between 22 and 60 years of age having femoral condyle and /or trochlea articular cartilage lesions who have failed conservative therapy, in addition to meeting other criteria. The study is designed to assess the safety and performance of Chondrograft™, and procedural goals include regrowth of the cartilage-bone matrix, improvement in knee function and pain, and delayed need for arthroplasty.
“Achieving Health Canada approval allows us to gain the clinical data needed to take a giant step forward towards design and execution of a large pivotal North American study” said Ben Holmes, CEO of Nanochon. “We have the utmost confidence in Dr. Abuzgaya and his clinical team at DBJS to help us recruit the right patients and execute the trial protocol that we have so carefully designed.”
While the first in human trial will take place in Canada, this represents just the first step towards a larger clinical program.
“Thousands of patients with chondral defects, that may evolve to osteoarthritis, live with a reduced quality of life. We are looking forward to the potential for a new treatment option and are excited to begin the Chondrograft™ study at DBJS”, states Dr. Joel Lobo. “The results of this study could open up a new surgical option, that is minimally invasive to address this unmet clinical need.”
Throughout the clinical trial design and regulatory approval process, Nanochon was supported by HN Clinical Consulting (Missouri). “Improving the lives of young adults and athletes by providing a solution to restore knee cartilage and hopefully help patients avoid costly knee replacement is a crucial therapy needed in the orthopedic world,” said Heather Neill, Founder and Principal. “We appreciate Health Canada’s thought-provoking questions throughout their review that helped us put forward the strongest application possible, which ultimately led to our approval.” HN Clinical Consulting will continue to support the full life cycle of Chondrograft™.
About HN Clinical Consulting
HN Clinical Consulting, LLC, brings a strategic focus to each project, keeping the goal of commercialization in sight. Building strong relationships with all stakeholders and maintaining a clinical strategic goal of project-oriented success to support long term Sponsor strategy/exit is key to development of clinical programs. The objective set is to stay ahead by planning for podium presence, publication, and reimbursement while designing the Clinical Investigational Plan.
About Nanochon
Nanochon is a biotech and medical device company focused on developing innovative orthopedic solutions. Chondrograft™ is a minimally-invasive implant that allows for immediate weight-bearing and motion – meaning less time spent recovering. The implant is based on research from the Tissue Engineering and Nanotechnology lab at the George Washington University, and is designed to integrate with healthy tissue over time, for maximum stability and benefit to the patient. Nanochon has the potential to deliver more successful and longer-lasting recovery for patients than current standard of care. Our mission is to develop a new approach to treat cartilage treatment so that the hundreds of thousands of young, active patients with joint damage can return to their lifestyles without undergoing costly and invasive short-term fixes.
www.nanochon.com
Contact
Rachel Offenburg, Chief of Strategy
rachel.offenburg@nanochon.com

 VIENNA, Va.–(BUSINESS WIRE)–CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the pricing of a best-efforts offering of 1,500,000 shares of its common stock. Each share of common stock is being sold at an offering price of $3.82 per share, priced at-the-market under NYSE American rules. Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, are expected to be approximately $5.7 million. The offering is expected to close on July 14, 2025, subject to satisfaction of customary closing conditions.
VIENNA, Va.–(BUSINESS WIRE)–CEL-SCI Corporation (“CEL-SCI” or the “Company”) (NYSE American: CVM), a clinical stage cancer immunotherapy company, today announced the pricing of a best-efforts offering of 1,500,000 shares of its common stock. Each share of common stock is being sold at an offering price of $3.82 per share, priced at-the-market under NYSE American rules. Total gross proceeds from the offering, before deducting the placement agent’s fees and other offering expenses, are expected to be approximately $5.7 million. The offering is expected to close on July 14, 2025, subject to satisfaction of customary closing conditions.



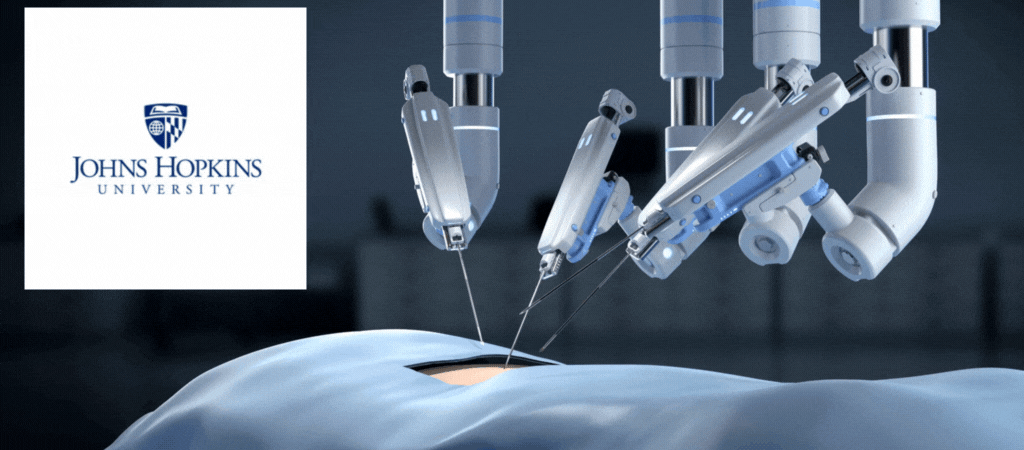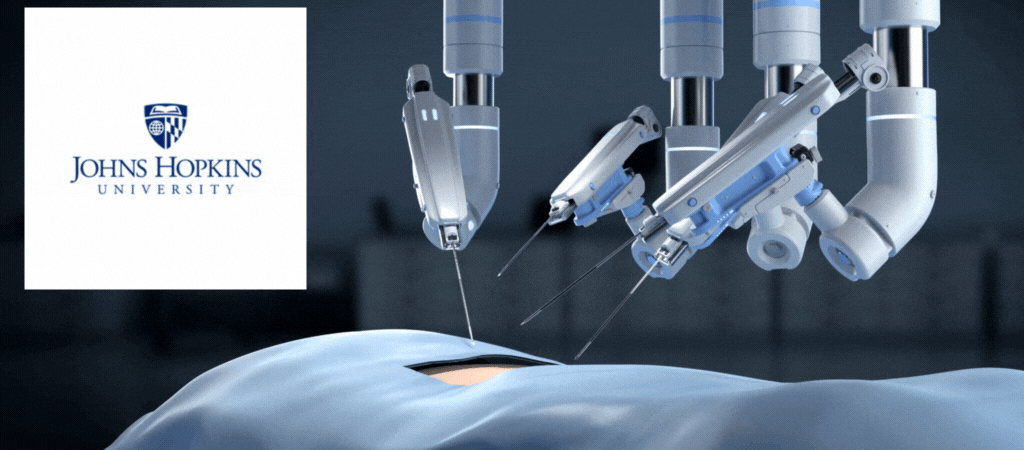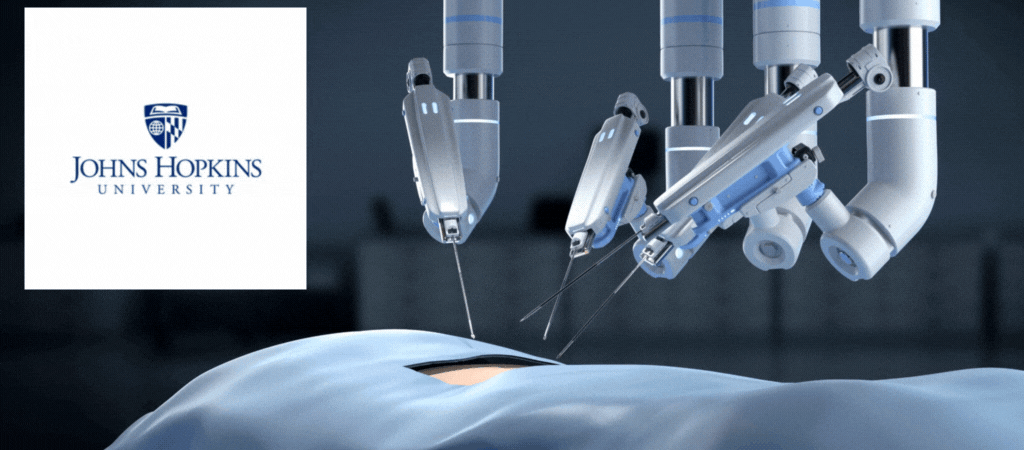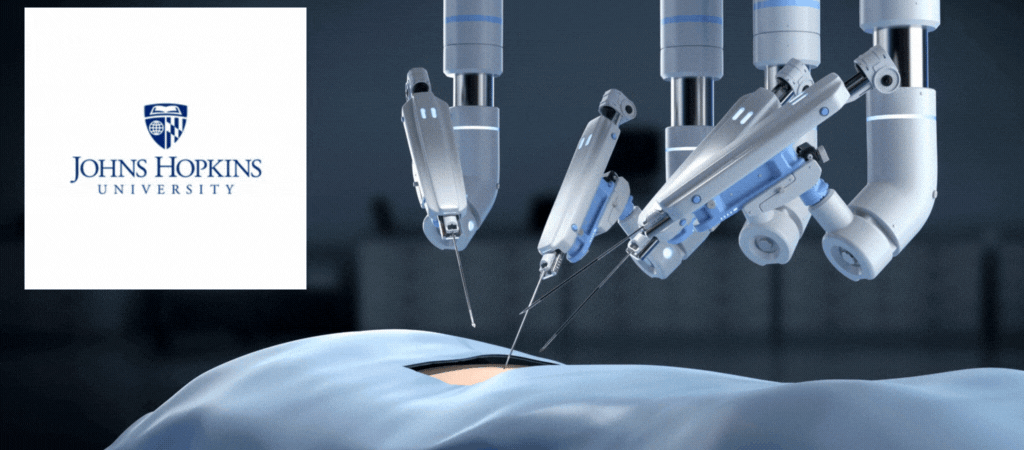
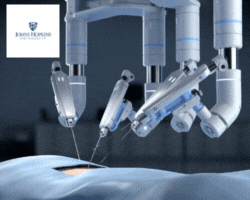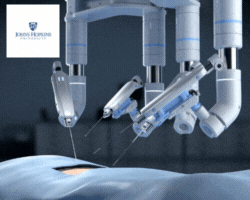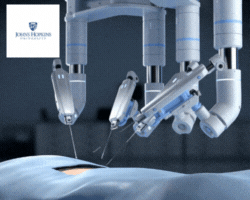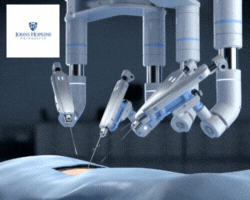 by Jill Rosen – A robot trained on videos of surgeries performed a lengthy phase of a gallbladder removal without human help. The robot operated for the first time on a lifelike patient, and during the operation, responded to and learned from voice commands from the team—like a novice surgeon working with a mentor.
by Jill Rosen – A robot trained on videos of surgeries performed a lengthy phase of a gallbladder removal without human help. The robot operated for the first time on a lifelike patient, and during the operation, responded to and learned from voice commands from the team—like a novice surgeon working with a mentor.
 Getting a biotech company off the ground takes more than science; it takes strategy, funding, and the right network.
Getting a biotech company off the ground takes more than science; it takes strategy, funding, and the right network. 
 GAITHERSBURG, Md., July 08, 2025 (GLOBE NEWSWIRE) — Emergent BioSolutions Inc. (NYSE: EBS) today announced a contract modification has been secured to deliver CNJ-016® [Vaccinia Immune Globulin Intravenous (Human)] (VIGIV) to the Administration for Strategic Preparedness and Response (ASPR), part of the U. S. Department of Health and Human Services (HHS), for smallpox preparedness. ASPR exercised an option from its existing 10-year contract (75A50119C00037) for additional doses of VIGIV, a treatment for complications due to smallpox vaccination.
GAITHERSBURG, Md., July 08, 2025 (GLOBE NEWSWIRE) — Emergent BioSolutions Inc. (NYSE: EBS) today announced a contract modification has been secured to deliver CNJ-016® [Vaccinia Immune Globulin Intravenous (Human)] (VIGIV) to the Administration for Strategic Preparedness and Response (ASPR), part of the U. S. Department of Health and Human Services (HHS), for smallpox preparedness. ASPR exercised an option from its existing 10-year contract (75A50119C00037) for additional doses of VIGIV, a treatment for complications due to smallpox vaccination.
 The Annual BioHealth Capital Region Investment Conference is the premier destination for discovering emerging companies that are redefining what’s possible in life sciences.
The Annual BioHealth Capital Region Investment Conference is the premier destination for discovering emerging companies that are redefining what’s possible in life sciences.
 ROCKVILLE, Md.–(BUSINESS WIRE)–The Maryland Tech Council (MTC), the largest technology and life sciences trade association in the state, today announced the launch of the Rural Technology Network, a new initiative to accelerate growth in rural Maryland’s technology ecosystem.
ROCKVILLE, Md.–(BUSINESS WIRE)–The Maryland Tech Council (MTC), the largest technology and life sciences trade association in the state, today announced the launch of the Rural Technology Network, a new initiative to accelerate growth in rural Maryland’s technology ecosystem.
 In this episode of BioTalk, Rich Bendis welcomes Dr. Helen Sabzevari, President and CEO of Precigen, to discuss the company’s cutting-edge science in gene and cell therapy. Dr. Sabzevari shares how Precigen’s unique AdenoVerse® platform has powered the development of PRGN-2012, a potential first-in-class therapeutic currently under FDA priority review for the treatment of adults with recurrent respiratory papillomatosis (RRP), a rare and devastating disease. She also highlights advances across Precigen’s broader pipeline in immuno-oncology and autoimmune disease and reflects on how Maryland’s BioHealth Capital Region has supported the company’s innovation and growth.
In this episode of BioTalk, Rich Bendis welcomes Dr. Helen Sabzevari, President and CEO of Precigen, to discuss the company’s cutting-edge science in gene and cell therapy. Dr. Sabzevari shares how Precigen’s unique AdenoVerse® platform has powered the development of PRGN-2012, a potential first-in-class therapeutic currently under FDA priority review for the treatment of adults with recurrent respiratory papillomatosis (RRP), a rare and devastating disease. She also highlights advances across Precigen’s broader pipeline in immuno-oncology and autoimmune disease and reflects on how Maryland’s BioHealth Capital Region has supported the company’s innovation and growth.