|
|
|

Wednesday, March 20, 2013, 04:30pm – 07:30pm
Join us on March 20 at Growlers for another BioBuzz Happy Hour
Join our sponsors, BioHealth Innovation, Inc. (BHI) and the Johns Hopkins University Center for Biotechnology Education, along with many others from our local biotech industry at another exciting BioBuzz event on March 20 from 4:30 – 7 p.m. in Gaithersburg. This month, we’re having our event a week early to accommodate BioBuzzers with kids in Montgomery County Public School system who will be on spring break the next week. We’re also holding the March BioBuzz event at a new location, Growlers in Old Towne Gaithersburg. We’re excited to see all of you soon, so please register today!
back to top 

Biopharmaceutical giant AstraZeneca announced today it plans to create a research and development center in Gaithersburg, adding 300 jobs at MedImmune’s current location.
MedImmune’s director of corporate public relations, Tracy Rossin, said no new buildings are planned at this point. Biotech company MedImmune, which is owned by AstraZeneca, currently houses AstraZeneca’s biologics programs, geared toward the creation of vaccines and medications.
back to top 

Every week we give you a countdown of the top five to 10 companies or organizations from one of our Lists publishing in our Friday paper. This week I present to you the top five “Venture capital firms investing the most in Maryland companies,” ranked by total amount invested in Maryland companies in 2012. This information was provided to us by the folks at MoneyTree Report by PricewaterhouseCoopers and the National Venture Capital Association based on data from Thomson Reuters.
back to top 
 
NOW IN ITS 7th YEAR!
University Startups Showcase and Conference 2013
March 20-22, 2013
Washington Convention Center, Washington, D.C.
“Corporate Venture Capital and University Startups: An Open Innovation Paradigm”
GETTING YOUR UNIVERSITY STARTUP FUNDED
2 WEEKS LEFT
REGISTRATION CLOSES: Friday March 15, 2013
(no onsite registrations)
Click here to register
or go to http://www.ncet2.org/index.php?option=com_content&view=article&id=543
back to top 

Rockville biotech Sequella inc. is looking to raise at least $20 million to advance its lead antibiotic candidate through clinical trials in drug-resistant tuberculosis and the stomach bacteria H. pylori.
If there ever was a time for the company to hit the gas pedal, it’s now. The resurgence of tuberculosis, especially in populous nations such as India and Russia, has brought what was thought of as a 19th century disease back into the spotlight. And the rise of multiple-drug-resistant strains of TB has made that fear very real in the mind of the U.S. consumer.
back to top 

People looking to start a small business in Montgomery County can have some of their questions answered at a series of seminars focusing on small business in Germantown.
Three seminars will be held between March, April and May by Score DC, a chapter of the U.S. Service Corps and Retired Executives, and will have mentors on hand to discuss successful business practices.
back to top 

As our April 5, 2013 receipt date approaches and you prepare your grant submission, please remember to work to register in all the required systems (DUNS, SAM, grants.gov, eRA Commons) in advance. These must all be complete before you can submit your grant application.
Company registration at SBA’s SBIR.gov is NOT required for submissions at this time. Solicitations issued after 1/28/2013 (not due dates for solicitations already on street prior to that date) will have instructions on how to register at SBA.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
Request for Applications (RFAs):
- RFA-OD-13-004: Lasker Clinical Research Scholars Program (Si2)
This FOA solicits applications for the Lasker Clinical Research Scholars Program for the purpose of supporting the research activities during the early stage careers of independent clinical researchers.
- RFA-RM-12-022: NIH Director’s Biomedical Research Workforce Innovation Award: Broadening Experiences
The purpose of this FOA is to seek, identify and support bold and innovative approaches to broaden graduate and postdoctoral training, such that training programs reflect the range of career options that trainees (regardless of funding source) ultimately may pursue and that are required for a robust biomedical, behavioral, social and clinical research enterprise. Collaborations with non-academic partners are encouraged to ensure that experts from a broad spectrum of research and research-related careers contribute to coursework, rotations, internships or other forms of exposure. This program will establish a new paradigm for graduate and postdoctoral training; awardee institutions will work together to define needs and share best practices.
- RFA-RM-13-001: Planning Grants for the NIH Building Infrastructure Leading to Diversity (BUILD) Initiative (P20)
The purpose of this Funding Opportunity Announcement (FOA) is to encourage institutions with expertise and innovative strategies for developing research and mentoring opportunities for undergraduate students from backgrounds underrepresented in biomedical research to submit applications for 6 month planning grants for the NIH Building Infrastructure Leading to Diversity (BUILD) initiative. The BUILD initiative aims to increase the diversity of the NIH-funded workforce by supporting collaborative programs that include novel approaches for enhancing undergraduate education, training, and mentorship, as well as infrastructure support and faculty development to facilitate those approaches.
- RFA-RM-13-002: Planning Grants for the NIH National Research Mentoring Network (NRMN) (P20)
The purpose of this Funding Opportunity Announcement (FOA) is to encourage organizations with experience in the mentorship of individuals underrepresented in the biomedical research workforce to submit planning grant applications for the NIH National Research Mentoring Network (NRMN). The NRMN will establish a nationwide consortium to provide networking and mentorship experiences for individuals from backgrounds underrepresented in biomedical research from the undergraduate to junior faculty level.
Program Announcement (PA):
- PAR-13-137: Bioengineering Research Grants (BRG) (R01)
The purpose of this funding opportunity announcement is to encourage collaborations between the life and physical sciences that: 1) apply a multidisciplinary bioengineering approach to the solution of a biomedical problem; and 2) integrate, optimize, validate, translate or otherwise accelerate the adoption of promising tools, methods and techniques for a specific research or clinical problem in basic, translational, or clinical science and practice. An application may propose design-directed, developmental, discovery-driven, or hypothesis-driven research and is appropriate for small teams applying an integrative approach that can increase our understanding of and solve problems in biological, clinical or translational science.
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Known for its ancient castles and seaside mosques, the Turkish city of Trabzon is looking to raise its profile on a new front—biotechnology.
For inspiration on expanding the Trapzon region’s high-tech and environmental-based industry, a foreign delegation arrived in Baltimore on Tuesday to meet with the Maryland Department of Business & Economic Development.
back to top 

At its annual meeting on March 8, the Maryland Business Incubation Association (MBIA) approved applications for membership for two new full incubator programs and one new associate program: The Harford Business Innovation Center, Betamore, and The Charles County Innovation Center (planning underway)—bringing current membership to 23 business incubators and innovation centers.
MBIA member organizations offer direct support to nearly 450 entrepreneurial ventures throughout the State from a wide variety of sectors. They foster entrepreneurship and contribute substantially to the Maryland economy through the creation of thousands of jobs and the generation of significant tax revenues.
back to top 

The financial future looks bright for students at the University of Maryland College Park.
Recent graduates from Maryland earn the second highest starting salary among grads from all 50 state flagship schools.
back to top 

In laboratory studies, Johns Hopkins researchers say they have found that stem cells from a patient’s own fat may have the potential to deliver new treatments directly into the brain after the surgical removal of a glioblastoma, the most common and aggressive form of brain tumor.
The investigators say so-called mesenchymal stem cells (MSCs) have an unexplained ability to seek out damaged cells, such as those involved in cancer, and may provide clinicians a new tool for accessing difficult-to-reach parts of the brain where cancer cells can hide and proliferate anew. The researchers say harvesting MSCs from fat is less invasive and less expensive than getting them from bone marrow, a more commonly studied method.
Results of the Johns Hopkins proof-of-principle study are described online in the journal PLOS ONE.
back to top 

Roughly three years after announcing that he was expanding his California bioscience incubator to San Antonio, InCube Labs founder Mir Imran may wish he had made the move even sooner.
InCube has found willing investors, as well as corporate and community support, in the nation’s seventh largest city. That has allowed the organization to adjust its expectations and strategies in a way that could prove to be hugely beneficial for the life sciences entity and for San Antonio long-term.
back to top 

New Enterprise Associates, a venture capital firm that’s invested some $13 billion in up-and-coming companies, has launched a brand-new design mentorship program to fuel innovation in the design industry. Called NEA Studio, the 12-week program will challenge five designers at a time.
Why the focus on design? “When a consumer gets a product, it’s usually because of the design of it,” said Dayna Grayson, an NEA partner, to Fast Company. “I feel like, if you’re really going to design a product and make it inherent at a company, it has to start at a founder level. So if the designer wants to be the founder, why not?”
back to top 

As part of the Wyle-led team, Lockheed Martin (NYSE: LMT) has been selected by NASA’s Johnson Space Center to provide biomedical, medical and health services in support of all human spaceflight programs. These services under the Human Health and Performance Contract (HHPC) monitor astronaut health and enable bioastronautics research that benefits life on Earth.
The potential contract value to Lockheed Martin is about $250 million over the expected 10-year life of the contract. Lockheed Martin is responsible for flight hardware development, facilitation of life sciences research conducted on the International Space Station (ISS), human factors engineering to optimize tools and experiments for astronauts in zero gravity, radiation analysis, space food development, flight/ground crew training, and life sciences data archival.
back to top 

As advances in genomics, molecular analysis, and data processing have propelled disease research forward, scientists and drug developers still face a formidable challenge: recruiting patients for their studies.
Genetic Alliance, a nonprofit that advocates for people with rare genetic disorders, is launching a new site called Reg4All that aims to entice more patients into clinical trials and disease research by giving them unprecedented privacy controls and greater say in how their data is used for research.
back to top 

After McKesson, Cerner, Allscripts, Greenway and athenahealth made news at HIMSS13 this past week with the launch of the CommonWell Health Alliance – putting aside their competitive instincts, for a moment, to pledge their common commitment to interoperability and data liquidity – Healthcare IT News spoke with McKesson CEO John Hammergren about the road ahead.
Joining Hammergren in the discussion were David McCallie, vice president, medical informatics at Cerner, and Arien Malec, vice president, data platform solutions for McKesson’s connectivity business, RelayHealth (and, in his former role at the Office of the National Coordinator for Health IT, the driving force behind the development of the Direct Project).
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

March 20-22
Washington Convention Center, Washington, D.C.

March 20
Growlers in Old Towne Gaithersburg
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

The Economic Alliance of Greater Baltimore (EAGB) and BioHealth Innovation (BHI) in collaboration with the Baltimore Business Journal are proud to announce the publication of the Central Maryland BioHealth Entrepreneur’s Resource and Financing Guide.
This Guide will be a compendium of resources to BioHealth innovators and entrepreneurs working to start and grow new companies in the region. A wealth of resources exists in Maryland to support emerging BioHealth companies, however, they are not always readily accessible or captured in one place. The Guide will compile information on financial resources, university facilities and programs, economic development programs, and existing federal laboratory facilities and programs and how to work with them.
back to top 
 Join us on March 20 at Growlers for another BioBuzz Happy Hour Join us on March 20 at Growlers for another BioBuzz Happy Hour
Join our sponsors, BioHealth Innovation, Inc. (BHI) and the Johns Hopkins University Center for Biotechnology Education, along with many others from our local biotech industry at another exciting BioBuzz event on March 20 from 4:30 – 7 p.m. in Gaithersburg. This month, we’re having our event a week early to accommodate BioBuzzers with kids in Montgomery County Public School system who will be on spring break the next week. We’re also holding the March BioBuzz event at a new location, Growlers in Old Towne Gaithersburg. We’re excited to see all of you soon, so please register today!
back to top 

Aronson’s Technology Industry Services Group is partnering with Rich Bendis and BioHealth Innovation (BHI) to help transform Central Maryland into a leading biohealth entrepreneurial and commercialization region.
“The central Maryland region is home to research organizations, government agencies, universities and companies with extensive biohealth experience and capabilities,” remarked Alan Langelli, Senior Manager in Aronson’s Technology Industry Services Group. He continued, “Aronson is very excited about the opportunity to partner with BHI and support its goal of helping Central Maryland become a leading biohealth entrepreneurial and commercialization region.”
back to top 
 BioHealth Innovation, Inc., (BHI) a non-profit organization which strives to facilitate the development of commercially viable health IT products and companies by connecting market relevant research assets to appropriate funding, management and markets, is seeking a Health Information Technology (IT) Entrepreneur-in-Residence. BioHealth Innovation, Inc., (BHI) a non-profit organization which strives to facilitate the development of commercially viable health IT products and companies by connecting market relevant research assets to appropriate funding, management and markets, is seeking a Health Information Technology (IT) Entrepreneur-in-Residence.
POSITION DESCRIPTION – Health IT Entrepreneur-in-Residence
The Health IT Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage health IT technologies, advise BHI on opportunities for new ventures, and build a portfolio commercially relevant health IT opportunities. The Health IT EIR influences the BHI organization by managing and providing information, intelligence and insights that drive critical business decisions. The Health IT EIR will work with early stage companies to launch and validate those companies while providing recommendations and insights on the direction of potential technologies. The Health IT EIR has the potential to also serve in a co-management role in a health IT accelerator.
back to top 

back to top 

Personal Genome Diagnostics Inc. (PGDx), a pioneer in conducting patient-specific analyses aimed at identifying genomic alterations in tumors, today announced a number of developments that will support its expanding business. The company licensed exclusive rights to Digital Karyotyping (DK), an important genome-mapping technology developed by the company’s founders at Johns Hopkins University. PGDx also announced that it is expanding into new facilities and has made a number of key hires, including Genzyme Oncology executive Antony Newton as Chief Commercial Officer.
back to top 

Johns Hopkins University generally does well on U.S. rankings of the top colleges.
But how does it stack up against institutions of higher learning from around the world? Not bad. It ranks No. 19 in the Times Higher Education World Reputation Rankings of the top 100 universities from around the globe. The University of Maryland, College Park also comes up on the list, at 95.
back to top 

The University of Maryland, College Park has cracked the top 100 global reputation ranking by a publication based in the United Kingdom, The Washington Post reported.
Times Higher Education shows that UMd. is in a group ranked 91-100, in a class with Monash University in Australia, Lund in Sweden, Bristol in the U.K., the Free University of Berlin and Texas A&M. The rankings were based on surveys of academics around the world. Johns Hopkins, in Baltimore, ranked No. 19, the highest from the local region. Harvard University was ranked No. 1.
back to top 

GlaxoSmithKline (GSK) has submitted its albiglutide once-weekly injection for type 2 diabetes to European regulators.
If approved the biologic treatment, which was submitted for US approval in January, will be marketed as Eperzan.
Albiglutide is, along with lupus treatment Benlysta and heart disease drug darapladib, one of a trio of drugs GSK has developed with Human Genome Sciences.
back to top 

Montgomery County-based Novavax is a biopharmaceutical company creating nanoparticle vaccines targeting a wide array of infectious diseases. In this new video, CEO Stanley Erck speaks about the different processes Novavax scientists are working on, and the overall momentum of the company. Mr. Erck also highlights the company’s partnerships with Path, LG Life Sciences, GE Healthcare, and Cadila Pharmaceuticals. Novavax is excited by the promising new data from clinical trials with its RSV vaccine candidate and pandemic influenza vaccine candidate, and by the potential of its technology platform. From discovery to commercialization, Novavax is positioned to develop the vaccines for tomorrow.
back to top 

A tax measure that would create a new, more lenient capital gains rate for tech investors and entrepreneurs cashing out their stock in the District is “going to be a focal point” for the Gray administration’s policy efforts this year, Mayor Vincent Gray told me in an interview Friday.
Gray, discussing his upcoming trip to the South by Southwest technology competition in Texas and his broader efforts to expand the District’s tech startup scene, acknowledged that resistance to the tax cut persists in the D.C. Council, which tabled the tax break last summer:
“I don’t get the sense that they’re any further along,” he said.
back to top 

Nevro Corp., a medical device company focused on improving pain relief in patients suffering from debilitating chronic pain, today announced it has completed a $48 million Series C financing round. The round was led by new investor Novo Ventures, joined by New Enterprise Associates (NEA) and Covidien Ventures. Existing investors participating in this financing round included Accuitive Medical Ventures (AMV), Bay City Capital, Johnson & Johnson Development Corporation (JJDC), Mayo Clinic, MPM Capital, and Three Arch Partners.
“We are excited to welcome premier investors Novo Ventures, NEA, and Covidien Ventures who share Nevro’s vision to be a leader in neuromodulation through continuous innovation”
back to top 

Washington, D.C., is known for its cut-throat politics and its power players.
What many do not know is that D.C. is now a hub for business innovation.
back to top 

Personalized medicine — the ability to tailor therapies to patients’ individual genetic characteristics — has long been the holy grail of the life sciences industry. The effort has produced a string of recent successes, including a host of drugs targeted to people with specific genetic profiles, the European approval of the world’s first gene therapy treatment, and a much-heralded leukemia treatment pioneered at Children’s Hospital of Philadelphia (CHOP) that uses tweaked versions of patients’ own cells to eliminate their cancer. While these advances are certainly exciting for patients, they raise a host of ethical, legal and financial challenges that people working in the field will need to address before personalized medicine can become a thriving business.
The challenges are so great, contends Wharton health care management professor Ezekiel J. Emanuel, that claims of a renaissance in medicine brought on by individualized approaches often seem hyperbolic. “Before we buy into this, we need to remember that almost every evaluation of what drives health care costs up points to new technologies,” says Emanuel, who is also a professor of medical ethics and health policy at Penn’s Perelman School of Medicine. “We need to be skeptical. We need to see the data before people buy into the idea that personalized medicine is going to produce cost savings and be so much better for the system.”
back to top 
 She sent her first tweet just after noon, and already Kathleen Sebelius (@Sebelius) has 2,000-plus followers. She sent her first tweet just after noon, and already Kathleen Sebelius (@Sebelius) has 2,000-plus followers.
The secretary of U.S. Health and Human Services is the latest federal health official to join Twitter and follows in the footsteps of colleagues Dr. Tom Frieden (@DrFriedenCDC), the director of the U.S. Centers for Disease Control and Prevention, and Dr. Francis Collins (@NIHDirector), director of the National Institutes of Health.
back to top 

Big news was made at HIMSS13 on Monday when, in an unprecedented collaboration, some health IT heavy-hitters joined forces in an effort to push the needle on interoperability.
In announcing the launch of the CommonWell Health Alliance, executives from Cerner, McKesson, Allscripts, athenahealth, Greenway and RelayHealth touted what they say is a first-of-its-kind organization: a collaboration of rival vendors, uniting to enable care integration and data liquidity.
back to top 
 
NOW IN ITS 7th YEAR!
University Startups Showcase and Conference 2013
March 20-22, 2013
Washington Convention Center, Washington, D.C.
“Corporate Venture Capital and University Startups: An Open Innovation Paradigm”
GETTING YOUR UNIVERSITY STARTUP FUNDED
2 WEEKS LEFT
REGISTRATION CLOSES: Friday March 15, 2013
(no onsite registrations)
Click here to register
[or go to http://www.ncet2.org/index.php?option=com_content&view=article&id=543]
back to top 
A l ittle more than a month ago, Naomi Fried, chief innovation officer at Boston Children’s Hospital, tweeted: that most healthcare mobile apps are built for consumers and focus on health and wellness, while only 20 percent are clinical apps. She wrote: “We need more #hospital apps.” Naturally, this caught our attention. ittle more than a month ago, Naomi Fried, chief innovation officer at Boston Children’s Hospital, tweeted: that most healthcare mobile apps are built for consumers and focus on health and wellness, while only 20 percent are clinical apps. She wrote: “We need more #hospital apps.” Naturally, this caught our attention.
“There’s a huge opportunity for mobile apps in the hospital environment,” Fried told MobiHealthNews in an interview ahead of the annual Healthcare Information and Management Systems Society (HIMSS) conference. Fried will be receiving a HIT Men & Women Award at HIMSS in New Orleans this week for being an up-and-coming innovator.
back to top 
 In 2009, President Barack Obama selected the Republic of Turkey as a pivotal port of call in his first trip abroad as the new President of the United States. That symbolic visit led to the launch of an initiative to boost trade and investment ties between the two strategic allies under what was called a “model partnership,” culminating in a $ 17 billion mutual trade volume. Following in the footsteps of the President, US Senator John Kerry will also visit Turkey in his first international tour as the new American Secretary of State. These visits further underline the significance of American-Turkish relations for this administration. As the 16th strongest economy in the world, Turkey is not only an important geopolitical ally, but also an increasingly valuable commercial partner. In 2009, President Barack Obama selected the Republic of Turkey as a pivotal port of call in his first trip abroad as the new President of the United States. That symbolic visit led to the launch of an initiative to boost trade and investment ties between the two strategic allies under what was called a “model partnership,” culminating in a $ 17 billion mutual trade volume. Following in the footsteps of the President, US Senator John Kerry will also visit Turkey in his first international tour as the new American Secretary of State. These visits further underline the significance of American-Turkish relations for this administration. As the 16th strongest economy in the world, Turkey is not only an important geopolitical ally, but also an increasingly valuable commercial partner.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today the formation of its Commercial Relevance Advisory Board (CRAB). Members of the CRAB represent diverse business and academic organizations from throughout the region, such as venture capital firm New Enterprise Associates, Ernst & Young, the Howard Hughes Medical Institute, the University of Maryland (UMD), Johns Hopkins University, and leading biotechnology companies.
The CRAB will assist BHI in evaluating market-relevant research and product development opportunities as well as help determine the commercial applicability of emerging entrepreneurs, companies and their science or technology. BHI will utilize feedback from CRAB members as part of its due diligence process for establishing formal client relationships and potentially future investments.
back to top 
 In partnership with BHI, the SBIR Resource Center(R) is offering a daylong seminar on How To Win SBIR Awards(SM) – at all federal agencies – with a primary emphasis on the National Institutes of Health (NIH). This event incorporates ALL of the necessary strategic planning, persuasive proposal writing, project planning/management and Federal project cost-accounting strategies to make your application successful. In partnership with BHI, the SBIR Resource Center(R) is offering a daylong seminar on How To Win SBIR Awards(SM) – at all federal agencies – with a primary emphasis on the National Institutes of Health (NIH). This event incorporates ALL of the necessary strategic planning, persuasive proposal writing, project planning/management and Federal project cost-accounting strategies to make your application successful.
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association with more than 400 life science and technology members employing more than 200,000 in the region, will honor Dr. James Barrett, a general partner in venture capital firm New Enterprise Associates (NEA), with its third annual Lifetime Achievement Award. Barrett will be presented the award at TCM’s Lifetime Achievement Gala, which is taking place March 6 at the Bethesda North Marriott Hotel and Conference Center.
The TCM Lifetime Achievement Award is given each year to a local individual who has gone above and beyond to serve the community at large over the course of his or her career. Recipients display commitment and leadership both in the field and within their company, fostering new ideas and encouraging creativity. The recipient also demonstrates generosity and compassion, making sure their work benefits others.
back to top 

The InvestMaryland Challenge has whittled down the 259 start-up companies that applied to the business competition to 33 semifinalists.
Only nine of those companies will move on to be finalists in the contest; three will win grand prizes of $100,000.
On March 5 the semifinalists will pitch their ideas to a panel of judges and complete a product demonstration. Grand prizes will be announced April 15.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
- NOT-HL-13-167: Notice of Intent to Publish a Funding Opportunity Announcement for Clinical Centers (CC)
The National Heart, Lung, and Blood Institute intends to publish a Funding Opportunity Announcement (FOA) to solicit applications for institutions to participate as Clinical Centers (CC) for the NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network, a new multi-center clinical trials network that will develop and test prevention or early treatment strategies for Acute Lung Injury. Read more.
- NOT-HL-13-168: Notice of Intent to Publish a Funding Opportunity Announcement for a Clinical Coordinating Center (CCC)
The National Heart, Lung, and Blood Institute intends to publish a Funding Opportunity Announcement (FOA) to solicit applications for institutions to serve as the Clinical Coordinating Center (CCC) for a new multi-center clinical trials network that will develop and test prevention or early treatment strategies for Acute Lung Injury. Read more.
- NOT-OD-13-043: NIH Operation Plan in the Event of a Sequestration
The NIH continues to operate under a Continuing Resolution as described in NOT-OD-13-002, and therefore all non-competing continuation awards are currently being funded at a level below that indicated on the most recent Notice of Award (generally up to 90% of the previously committed level). Final levels of FY 2013 funding may be reduced by a sequestration. Despite the potential for reduced funding, the NIH remains committed to our mission to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability. Read more.
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 
 The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets. The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets.
back to top 

Richard Bendis will speak at the Translational Research Forum during the 2013 BIO International Convention in Chicago. During the forum, speakers will address issues with current translational research models, explore new funding and collaboration opportunities, evaluate how to apply them to federal, academic, and private institutions. The session will take place Monday, April 22, from 4:35 p.m. – 5:25 p.m.
More Information
back to top 
 On a recent business trip to China, I was automatically assumed to be an interpreter or assistant because I was helping with communication on both sides. I had to assert myself and remind my colleagues that I, too, had original thoughts to contribute. This small example shines light on the issue of how Asian women are often perceived. We are seen as competent and hard-working, partly due to stereotypes, but not necessarily powerful or influential. In fact, projecting an image of power can invoke some resentment and cause discomfort. A man I once supervised admitted to me that he was not used to having a woman as manager. At least he was honest, which made it easier to work out the problem. On a recent business trip to China, I was automatically assumed to be an interpreter or assistant because I was helping with communication on both sides. I had to assert myself and remind my colleagues that I, too, had original thoughts to contribute. This small example shines light on the issue of how Asian women are often perceived. We are seen as competent and hard-working, partly due to stereotypes, but not necessarily powerful or influential. In fact, projecting an image of power can invoke some resentment and cause discomfort. A man I once supervised admitted to me that he was not used to having a woman as manager. At least he was honest, which made it easier to work out the problem.
back to top 

Johns Hopkins’ John Wong, Ph.D., has won a BioMaryland LIFE Award, and Ronald Berger, M.D., Ph.D., and Hien Nguyen, M.D., were awarded funds from the Abell Foundation, the researchers learned last week. Each of the winners will receive $50,000 to help develop their discoveries for clinical use.
The prizes were awarded as part of the annual Joint Meeting of the Johns Hopkins Alliance for Science and Technology Development and the University of Maryland, Baltimore Commercial Advisory Board on Feb. 19. The meeting was attended by more than 150 venture capitalists, seasoned biotech entrepreneurs and business development executives from the biopharma industry. Judging committees evaluated presentations from two dozen university researchers before selecting the winners. The aim of the awards is to speed the translation of promising research into commercial application.
back to top 

New technology from the University of Maryland (UM) could potentially provide a five-minute diagnostic test and a vaccine for tough-to-treat Staphylococcus aureus infections, including the antibiotic-resistant MRSA, often called a “super bug,” says inventor Mark Shirtliff, PhD, an associate professor at the UM School of Dentistry in Baltimore.
Shirtliff is the winner of the 2013 BioMaryland LIFE (Leading Innovative Faculty Entrepreneurs) Prize for the most promising technology from the University as awarded by a judging panel at the annual joint meeting of the UM Baltimore Commercial Advisory Board and the Johns Hopkins University (JHU) Alliance for Science and Technology Development.
back to top 

Venture investment in health care cratered in 2012. While others cut back, SR One charged ahead.
The venture arm of GlaxoSmithKline formed in 1985 and has steadily invested $30 million to $50 million annually. Seeing strong prospects at a time when many conventional venture firms had to sit it out, SR One invested more than $50 million last year, making eight new deals and eight follow-on investments, said Jens Eckstein, its president.
back to top 

If you need an effective drug today for age-related macular degeneration, the leading cause of blindness in the elderly, you need to get an injection at the back of the eye.
Waltham, MA-based Kala Pharmaceuticals believes it may be able to get the drug where it needs to go, without sticking a needle in your eye.
back to top 

In 2009, President Barack Obama selected the Republic of Turkey as a pivotal port of call in his first trip abroad as the new President of the United States. That symbolic visit led to the launch of an initiative to boost trade and investment ties between the two strategic allies under what was called a “model partnership,” culminating in a $ 17 billion mutual trade volume. Following in the footsteps of the President, US Senator John Kerry will also visit Turkey in his first international tour as the new American Secretary of State. These visits further underline the significance of American-Turkish relations for this administration. As the 16th strongest economy in the world, Turkey is not only an important geopolitical ally, but also an increasingly valuable commercial partner.
back to top 

GlaxoSmithKline encountered some stiff industry headwinds when it pledged to open up its data vault to outside investigators. But as of today it has a high-profile convert on its side. The biopharma giant Roche ($RHHBY) has agreed to follow in GSK’s ($GSK) footsteps, saying that it will work with an independent group which will be charged with sorting out and approving requests for access to anonymized clinical trial data for all approved products. If regulators can’t provide the data, says Roche, then the company will make it available.
“We understand and support calls for our industry to be more transparent about clinical trial data with the aim of meeting the best interests of patients and medicine,” said Daniel O’Day, chief operating officer of Roche Pharma. “At the same time, we firmly believe that health authorities need to remain the gatekeeper for drug assessment and approval. We believe we have found a way in which patient data can be provided to third party researchers in a legitimate environment that ensures patient confidentiality and avoids the risk of publishing misleading results or giving rise to public health scares and consequences.”
back to top 

Government contractors across the U.S. have found themselves in a holding pattern over the past few years, adapting to constant delays and waiting for economic uncertainty to be resolved. Between continuing resolutions, the debt ceiling crisis, and the threat of sequestration, government contracting companies have struggled to find new ways to be successful in the face of change.
According to a recent white paper by the Professional Services Council, “between fiscal years 2011 and 2012, federal spending on service contracts dropped more than $5 billion, which translates directly into the elimination of tens of thousands of contractor positions across the nation.” This, combined with a rise in the award of lowest-priced technically acceptable contracts, the delay in the award of new contacts and shorter term task orders under existing contract vehicles are all signs that a storm is brewing. Increased audits and investigations into contracting fraud waste and abuse, the increase in more regulations and compliance issues, and the Federal Strategic Sourcing initiative are clear evidence of this prevailing wind.
back to top 

A little more than a year ago the American Heart Association launched an accelerator to fund biotechnolgy and medical device startups to fill a crucial gap in research funding caused by the increasing hesitance of investors to risk support on early-stage innovation. Now, it’s getting ready for a $2 million fundraising round to invest in two companies by the end of the year. It’s also working with sister organizations and mission-driven investment organizations to identify areas of common interest.
In a phone interview with MedCity News, Ross Tonkens, the director of the Science & Technology Accelerator, and Major Gifts Officer Mark Germano said they’re forming a group of donors who can provide expertise to screen applications. These donors have investment backgrounds steeped in biotechnology, drug development and medical devices. They also have expertise in legal issues, commercialization, IP, regulatory, clinical trial design and conduct issues. In a lot of cases, these are people who have had personal or close contact with people who have had cardiovascular disease or a stroke and want to see things move from the [lab] bench to bedside.
back to top 
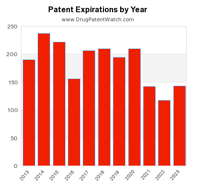
This live infographic dynamically shows the number of pending patent expirations by year for the next ten years.
For more infographics, see the DrugPatentWatch Pharmaceutical Innovation Infographics.
back to top 
 
NOW IN ITS 7th YEAR!
University Startups Showcase and Conference 2013
March 20-22, 2013
Washington Convention Center, Washington, D.C.
“Corporate Venture Capital and University Startups: An Open Innovation Paradigm”
GETTING YOUR UNIVERSITY STARTUP FUNDED
2 WEEKS LEFT
REGISTRATION CLOSES: Friday March 15, 2013
(no onsite registrations)
Click here to register
[or go to http://www.ncet2.org/index.php?option=com_content&view=article&id=543]
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|

Psyadon Pharmaceuticals has spent nine months working to enroll the 18 patients it needs for its phase 3 clinical trial involving its treatment for Tourette syndrome.
The Germantown company’s candidate, ecopipam, also targets Lesch-Nyhan disease, a genetic disorder that affects as many as 1,000 Americans, disrupting their ability to walk and causing self-mutilation.
Although Psyadon usually is not directly involved in its clinical trials — it usually uses companies called contract research organizations, which conduct trials for drug makers — it often tracks disease-related patient advocacy groups and sometimes uses this information to raise awareness of the trial, said CEO Richard Chipkin.
back to top 

The University of Maryland’s Maryland Industrial Partnerships (MIPS) program today announces it is awarding $4.7 million to Maryland university researchers to help 16 local companies develop technology products.
The projects, which team companies with universities across the state, include gene-silencing for cancer treatment, a new cardiovascular diagnostic device, advanced chemical detection, distributed heating and cooling, fertilizers and soil amendments made from both fish waste and other mixtures, agricultural stormwater treatment, an heirloom tomato juice production system, temperature-detecting gel, a drug to treat lung fibrosis, advanced oyster seeding system, electronic baseball home plate, mobile solar milk chiller, and both a vehicle and sensor technology for inspecting bridges.
back to top 

The young Leiden-based biotech company Mimetas has closed an agreement with a regional development company from the state of Maryland, USA. Biohealth Innovation Inc., in which regional governments, universities and companies collaborate, will assist in setting up a US branch to find customers and business partners for Mimetas in the USA.
back to top 
 
Richard Bendis will be a speaker at the South by Southwest (SXSW) Conference which takes place from March 8-17 in Austin, Texas. SXSW is a set of film, interactive, and music festivals which occur every year in March. Mr. Bendis will be speaking on March 9th at a session titled “Entrepreneurs in Residence: Not Just for VCs.” In his presentation, Richard will introduce the BioHealth Innovation, Inc. EIR program and speak about some of the commercialization challenges being addressed creatively by the biohealth community in Maryland.
back to top 
 
In partnership with BHI, the SBIR Resource Center(R) is offering a daylong seminar on How To Win SBIR Awards(SM) – at all federal agencies – with a primary emphasis on the National Institutes of Health (NIH). This event incorporates ALL of the necessary strategic planning, persuasive proposal writing, project planning/management and Federal project cost-accounting strategies to make your application successful. Walk away with a deep understanding of what it takes to win and an ability to customize SBIR/STTR win strategies for specific projects. Get equipped to evoke positive responses in SBIR/STTR proposal evaluators (very different from all other programs).
WHEN: Tuesday, 5 March 2013, 8:30am – 6:00pm (45 min. lunch break)
WHERE: Rockville Economic Development, Inc.95 Monroe Street – Rockville, MD 20850
(short walk from Rockville Metro Sta.)
301-315-8096 (location phone)
REGISTRATION: or call 410-315-8101 OR email the SBIR Center; Class limited to 18-20 participants
TUITION: $325 ($375 after February 25) — satisfaction assured or tuition is returned
DISCOUNT: BioHealth Innovation may underwrite $175 of the tuition for select biohealth companies through its Commercial Relevance Program.
To inquire about the discount, Contact Ethan Byler
back to top 

Life sciences research is a strong economic driver, even if it leads to few patents
As the economy continues to flounder, many cities are looking for ways to replicate Silicon Valley’s financial success. When seeking to catch the magic of those biggest successes — Apple, Google and Facebook — the word “innovation” gets thrown around frequently. And as intellectual property is taking on a larger and larger role in how companies do business in the Bay Area, many have equated innovation with patents.
A recent Sun article about innovation in Baltimore and Maryland focused on just that. It lamented that the Baltimore metro area came in 116th out of 360 metro areas for the number of patent applications per capita, and that the number of patents granted to Baltimoreans remained flat over the past decade. The article seemed to suggest that this lack of intellectual property growth was at least partially responsible for Baltimore’s lack of job growth.
back to top 
POSITION DESCRIPTION – Entrepreneur-in-Residence
 The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets. The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets.
back to top 

University of Maryland, College Park and two other East Coast schools will share $3.75 million from the National Science Foundation to develop a regional hub for turning university research into marketable products and services.
University of Maryland will be working with George Washington University and Virginia Tech to create an Innovation Corps for the mid-Atlantic region. The initiative will aim to draw out the best research ideas from students and faculty members and bring them to the commercial market.
back to top 

Rare Disease Day, held each year on February 28, was established to raise awareness about the estimated 7,000 rare diseases that affect about 25 million Americans. To mark the occasion in 2013, the NIH will host a free, two-day public event beginning on this day to focus on rare diseases research and advocacy activities supported by several government agencies.
The National Center for Advancing Translational Sciences (NCATS) Office of Rare Diseases Research (ORDR) and the NIH Clinical Center are organizing and hosting the event. Others involved include the U.S. Food and Drug Administration and Agency for Healthcare Research Quality, and patient organizations, such as the Genetic Alliance and National Organization for Rare Disorders. Register and learn more at https://events-support.com/events/Rare_Disease_Day .
back to top 

Johns Hopkins University Applied Physics Laboratory has won a potential 10-year, $4,904,853,263 U.S. Navy for research, development and engineering work throughout the Defense Department.
The contract includes a five-year option for review and approval by the assistant Navy secretary for research, development and acquisition and the assistant defense secretary for research and evaluation, the Defense Department said Feb. 15.
back to top 

McLean-based Science Applications International Corp., which announced plans in August to split off part of its operations into a separate publicly traded company, has decided on a name for the new business.
The spinoff, which will focus on national security, health and engineering, will be called Leidos.
back to top 
Leaders of several Richmond-area biotechnology-related companies said Thursday that they foresee personalized medicine as a major force driving the industry’s growth, but access to capital for small firms with good ideas remains a challenge.
“Life sciences is really the big, huge growth industry,” said Mike Grisham, the chief executive officer of GPB Scientific, a Richmond-based company focused on using microchip technology in health and life-science research.
back to top 

Some of Silicon Valley’s most prominent billionaires are making a big push to guide the tech world’s entrepreneurs into biotech.
Backing the Breakthrough Prize in Life Sciences are Yuri Milner; Sergey Brin and Anne Wojcicki; and Mark Zuckerberg and Priscilla Chan.
Fittingly, they’re making the announcement at the University of California at San Francisco’s Genentech Hall, a building named after one of the Bay Area’s biotech standouts.
back to top 

The National Science Foundation said today that it will fund a major expansion of its Innovation Corps program, an effort to teach NSF-funded university researchers how to build profitable startups around their technologies.
In its initial stages, the two-year-old “I-Corps” program has been flying researchers to Stanford University, the University of Michigan, and Georgia Tech for prototype versions of the “Lean Launchpad” course originally developed at Stanford by serial entrepreneur and startup guru Steve Blank. Now the program is spreading to nine more universities, which have been singled out for three-year grants totaling $11.2 million.
back to top 
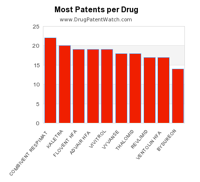
This chart shows the pharmaceutical drugs with the most patents still in force.
The drugs with the most active patents are COMBIVENT RESPIMAT, KALETRA, ADVAIR HFA,FLOVENT HFA, VIVITROL, THALOMID, VYVANSE, REVLIMID, VENTOLIN HFA, andCHILDREN’S ALLEGRA ALLERGY.
back to top 
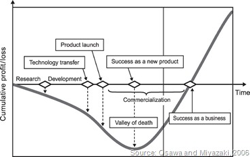
The “valley of death” is a common term in the startup world, referring to the difficulty of covering the negative cash flow in the early stages of a startup, before their new product or service is bringing in revenue from real customers. I often get asked about the real alternatives to bridge this valley, and there are some good ones I will outline here.
According to a Gompers and Lerner study, the challenge is very real, with 90% of new ventures that don’t attract investors failing within the first three years. The problem is that professional investors (Angels and Venture Capital) want a proven business model before they invest, ready to scale, rather than the more risky research and development efforts.
back to top 

The Association of University Research Parks (AURP), the world’s leading network of university research, science and technology park professionals, invites you to share your knowledge, expertise and experience by presenting at the 2013 International Conference in Philadelphia, Pennsylvania. This year’s theme is Inventing the Future.
Proven engines for economic growth and development, university research parks influence their communities in significant ways. AURP’s 2013 annual conference, hosted by The University City Science Center, will feature experts who will examine university research park best practices and the strategies which will develop a knowledge-based economy by increasing ties between university, research parks, government, and industry partners.
Interesting approaches and creative solutions to challenges surrounding this topic are sought for presentations.
back to top 

The health care industry is undergoing major surgery. At the center of these operations is Rock Health, a startup accelerator dedicated to the intersection of healthcare and technology. Today, at a demo event at the University of San Francisco, 14 startups presented their ideas on how to transform and improve healthcare in the U.S..
Dr. Aenor Sawyer, an associate clinical professor at UCSF, said during her opening remarks that these companies are changing “how we take care of patients and how patients take care of themselves.” Whether it is managing secondary care, untangling the confusing labyrinth of insurance, or encouraging healthy lifestyle habits, these startups are holding the scalpels.
back to top 

San Francisco’s Rock Health startup accelerator held its fourth semi-annual Demo Day at UCSF’s Genentech Hall Wednesday afternoon. Investors and journalists heard pitches from 14 startups working to introduce new health-related services for consumers and new ways to improve the efficiency of the U.S. healthcare system.
On the consumer side, one intriguing presenter was Beam Technologies, which is building a toothbrush embedded with motion sensors to detect how long a person has been brushing. A Bluetooth radio sends the data to a smartphone app. (Perhaps it should have been called the Bluetoothbrush.)
back to top 

Montgomery officials are under no illusions about the county’s image among the Washington region’s young: boring.
“We’re a little sleepy,” said County Council member Roger Berliner (D-Potomac-Bethesda). “We go to bed early.”
For all its prosperity and family-friendly suburban appeal, Montgomery is in the throes of a midlife crisis. That angst has led to a new item at the top of the public policy agenda: a yearning to be hip.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

March 5
Rockville Economic Development, Inc. – Rockville, MD

March 20-22
Washington Convention Center, Washington, D.C.

May 13-16
Gaylord National Hotel & Convention Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:
 |
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
|

Montgomery officials are under no illusions about the county’s image among the Washington region’s young: boring.
“We’re a little sleepy,” said County Council member Roger Berliner (D-Potomac-Bethesda). “We go to bed early.”
For all its prosperity and family-friendly suburban appeal, Montgomery is in the throes of a midlife crisis. That angst has led to a new item at the top of the public policy agenda: a yearning to be hip.
back to top 

Sen. Barbara Mikulski (D) dropped by Rockville on Monday for lunch with the Montgomery County Council, where much of the hour-long meeting focused on the looming federal government sequester.
Mikulski said she realized the effect across-the-board spending cuts and furloughs of federal workers could have on the county, home to 17 agencies, 32,000 employees and the many contracting firms that work with those agencies. There has been little recent movement on avoiding the sequester on Capitol Hill.
back to top 
POSITION DESCRIPTION – Entrepreneur-in-Residence
 The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets. The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets.
back to top 

The Department of Health and Human Services SBIR/STTR grant solicitation aimed at supporting small business innovation research is now available.
Through the PHS 2013-02 SBIR/STTR Omnibus Solicitation, U.S. small businesses are encouraged to submit investigator-initiated SBIR/STTR grant applications in response to a variety of identified topics related to the National Institutes of Health, the Centers for Disease Control and Prevention, the Food and Drug Administration and the Administration for Children and Families.
The SBIR/STTR Reauthorization Act of 2011 and the recently released SBIR and STTR policy directives have brought about numerous – and often times, complicated – changes. In an effort to keep the small business research community aware of the impending modifications, the NIH has set up a new website providing a detailed overview of its implementation plan. In addition, HHS intends to revise or re-issue the Omnibus solicitation later this year. To stay informed, download a copy of the current solicitation and request to be updated as changes are made.
back to top 

The National Cancer Institute (NCI) Small Business Innovation Research (SBIR) Program has announced $10M in funding for up to 10 new awards in FY2013.
The Phase IIB Bridge Award is designed to support the next stage of development for promising NIH-funded SBIR Phase II projects in the areas of cancer therapeutics, imaging technologies, diagnostics and prognostics, or interventional devices.
back to top 
 
In partnership with BHI, the SBIR Resource Center(R) is offering a daylong seminar on How To Win SBIR Awards(SM) – at all federal agencies – with a primary emphasis on the National Institutes of Health (NIH). This event incorporates ALL of the necessary strategic planning, persuasive proposal writing, project planning/management and Federal project cost-accounting strategies to make your application successful. Walk away with a deep understanding of what it takes to win and an ability to customize SBIR/STTR win strategies for specific projects. Get equipped to evoke positive responses in SBIR/STTR proposal evaluators (very different from all other programs).
WHEN: Tuesday, 5 March 2013, 8:30am – 6:00pm (45 min. lunch break)
WHERE: Rockville Economic Development, Inc.95 Monroe Street – Rockville, MD 20850
(short walk from Rockville Metro Sta.)
301-315-8096 (location phone)
REGISTRATION: or call 410-315-8101 OR email the SBIR Center; Class limited to 18-20 participants
TUITION: $325 ($375 after February 25) — satisfaction assured or tuition is returned
DISCOUNT: BioHealth Innovation may underwrite $175 of the tuition for select biohealth companies through its Commercial Relevance Program.
To inquire about the discount, Contact Ethan Byler
back to top 

Dominick Murray has been unanimously confirmed by the Senate Executive Nominations Committee to be the next head of the state’s economic development office.
Murray is scheduled to be sworn in to the role of secretary of the Maryland Department of Business and Economic Development by Gov. Martin O’Malley on Feb. 21.
back to top 

United Therapeutics Corporation announced today that the U.S. Food and Drug Administration (FDA) has acknowledged the resubmission of the new drug application (NDA) for treprostinil diolamine extended release tablets (oral treprostinil) for the treatment of pulmonary arterial hypertension. The FDA classified the resubmission as a complete, class 1 response to FDA’s October 23, 2012 complete response letter and the FDA set a user fee goal date of March 31, 2013.
back to top 
 Diagnostic products maker Qiagen NV said Wednesday that it will work with Eli Lilly and Co. to develop new tests that could identify patients who could be helped by Lilly’s drugs. Diagnostic products maker Qiagen NV said Wednesday that it will work with Eli Lilly and Co. to develop new tests that could identify patients who could be helped by Lilly’s drugs.
The companies did not disclose terms of the new collaboration, but described it as a “broad” partnership that will cover “all therapeutic areas.”
back to top 

The Cambridge Innovation Center, a longtime fixture of the Boston-area startup scene, is expanding its entrepreneur-friendly office space business to new cities—just as it continues to build a larger footprint in its hometown.
The CIC, which rents office space and related services to more than 500 companies in seven floors of a building near MIT, has been advertising for a general manager at a new Baltimore location. And CEO Tim Rowe says that’s not the only place the CIC is eyeing for a possible expansion.
back to top 

The Maryland Department of Business and Economic Development said 71 startups that entered its InvestMaryland Challenge have been selected from more than 250 applications to advance to the competition’s next round.
Eventually, three companies will win $100,000 and a chance to pitch their business to potential investors.The $100,000 prizes will be awarded in three categories: life sciences, information technology and general business.
back to top 

Emergent BioSolutions Inc. (NYSE: EBS) announced today that a member of the company’s senior management team will provide a corporate overview presentation at the Cowen and Company 33rd Annual Health Care Conference in Boston on Monday, March 4, 2013 at 3:30PM Eastern.
A webcast of this presentation will be available both live and by replay, accessible from the Emergent website www.emergentbiosolutions.com under “Investors”.
back to top 

On Friday, the Department of Defense announced that it has awarded The Johns Hopkins University Applied Physics Laboratory a five-year, sole source, cost-plus-fixed-fee, indefinite-delivery/indefinite-quantity task order contract to conduct research, development, engineering, and test and evaluation work for programs “throughout the Department of Defense.”
The contract envisions Hopkins scientists performing up to 11,964,743 staff-hours’ worth of research and development work through September 2017. Work would be performed in “core competency” areas such as “strategic systems test and evaluation; submarine security and survivability; space science and engineering; combat systems and guided missiles; theater air defense and power projection; and information technology (C4ISR/IO), simulation, modeling, and operations analysis.”
back to top 

A piece in Forbes this week calls attention to a recent trend in technology commercialization at universities: the use of crowdfunding.
The article focused on a collaboration between the University of Utah’s Technology Commercialization Office and the crowdfunding site RocketHub, which resulted in the University Tech Vault, a portal specifically for projects that come out of the university.
back to top 

As Valentine’s Day approaches the occasion begs the question: what does it take for two companies in the biopharmaceutical industry to merge? Of all the things that could come between them, how do a biotech startup and suitable partner find each other in this crazy, mixed up world?
Like any good marriage, the reasons that bring a couple together span of a good merger is more than meets the eye. The companies involved share similar goals and work hard to ensure the union endures. But there are all sorts of things That was the gist of an insightful panel discussion at the BIO CEO conference in New York. Among the panelists were: Michael Margolis, a managing director with ROTH Capital Partners, Effie Toshav, partner with Fenwick & West H.Thomas Watkins, former president and CEO of Human Genome Sciences until it was acquired by GlaxoSmithKline, Michael Gilman, a senior vice president at Biogen Idec and Corrine Epperly, the director of strategy, alliances and transactions at Bristol Myers-Squibb (NYSE: BMS)
back to top 
For years, U.S. life-sciences startups have sought to avoid some of the problems in their industry–including a scarcity of investment funding and a sometimes-daunting regulatory process–by raising funding or commercializing overseas.
Nowadays, foreign organizations and governments are the ones making the overtures, hoping that American life-sciences companies can create jobs and stimulate the life-sciences industries in their countries.
back to top 

The steady departure of pharmaceutical industry jobs in recent years has helped other states, but hurt the standing of the nation’s medicine chest. As more companies take root in far-flung locations, the New Jersey and New York City region has dropped significantly in the national ranking of life sciences markets, according to a recent report on commercial real estate.
Last year, the region slipped to seventh place among metropolitan life sciences clusters from second place in 2011, according to the latest annual report from Jones Lang LaSalle, the commercial real estate firm. The reasons cited for the slide: ongoing consolidation following big mergers and the simultaneous efforts among such cities as San Diego to offer competitive environments.
back to top 
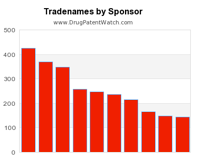
This chart shows the pharmaceutical companies with the most branded drugs.
The companies with the most drugs are Watson Labs, Hospira, Sandoz, Baxter Hlthcare,Mylan, Teva, B Braun, Roxane, Novartis, and Ivax Sub Teva Pharms.
back to top 

Innovate Health Tech NYC invites software and hardware developers and other innovators living or working in New York City to create new commercially viable technologies that solve urgent health care problems. Individuals, teams, and companies with 10 or fewer employes can compete, and will be required to demonstrate a functioning prototype of a pre-commercial technology in their submission. Technologies may include, but are not limited to, healthcare analytics tools, clinical workflow management tools, mobile health applications, and wireless health monitoring devices. Contestants are encouraged, but not required, to address healthcare priority areas identified by New York City.
back to top 

PILOT Health Tech NYC is an exciting new program which provides funding of up to $100,000 each to 10 innovative pilot projects to take place in New York City. The program seeks to match early-stage healthcare technology companies (‘innovators’) with key NYC healthcare service organizations or individuals (‘hosts’), including hospitals, physician clinics, payors, pharma companies, and nursing associations. Each pilot project will be focused on addressing defined needs of the healthcare industry and testing a technology prototype in a healthcare setting for a period of approximately 3-6 months.
back to top 

The Rice University Business Plan Competition is the world’s richest and largest graduate-level business plan competition. It is hosted and organized by the Rice Alliance for Technology and Entrepreneurship, Rice University’s nationally recognized entrepreneurship initiative, and the Jesse H. Jones Graduate School of Business, the #4 Best U.S. Graduate Entrepreneurship program per the Princeton Review.
In its 13th year, 42 teams from around the world will compete on campus April 11-13, 2013 for more than an expected $1 million + in cash and prizes in front of over 250 judges, primarily venture capitalists and other investors. More than 1200 teams applied to compete in 2012 and the competition was supported by more than 130 sponsors. More than 133 past competitors have successfully launched their business after competing at Rice, are still in business today, and have raised more than $480 million in funding.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

March 5
Rockville Economic Development, Inc. – Rockville, MD

March 20-22
Washington Convention Center, Washington, D.C.

May 13-16
Gaylord National Hotel & Convention Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today the appointment of GenVec, Inc. President and Chief Executive Officer Cynthia L. Collins to its Board of Directors. BHI also announced that BHI Founding Board Member Jerry Parrott, formerly with Human Genome Sciences, has stepped down from his board seat but will continue to remain active on BHI’s Commercial Relevance Advisory Board.
“Cindy’s addition to the BHI Board of Directors provides us with access to and perspective from another talented biopharma leader from this region,” said Scott Carmer, BioHealth Innovation, Inc. Chairman of the Board and Executive Vice President of Commercial Operations at MedImmune. “Her tremendous depth and breadth of experience across the industry, particularly with regard to diagnostics as well as therapeutic areas ranging from antivirals to oncology, will be an asset to the companies we work with who are seeking to commercialize biohealth innovations.
back to top 

Lets collaborate on building a vibrant biotech (or any other) community! Nice chestnut, but how does one design a community that functions across industries, geographies, support organizations, academic institutions and federal labs, each with very different missions and views of their (and others’) roles? How do you find a shared vision for these variant groups, one that drives growth for the greater region and doesn’t cause the players for fight over each opportunity as if it is the last scrap of possibility we’ll see? How do you prevent such a vision from becoming another dusty whitepaper, where behaviors weren’t aligned to make it happen? I had a chance to discuss how such a collaboration should be designed with Rich Bendis, President & CEO of BioHealth Innovation (BHI), an organization which spans from Rockville to Baltimore.
back to top 
POSITION DESCRIPTION – Entrepreneur-in-Residence
 The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets. The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets.
back to top 
 Whether you are a budding life science entrepreneur or potential investor, this event should be of interest to you. Multiple speakers explain how to acquire funding from Venture Capitalists and other investors. Whether you are a budding life science entrepreneur or potential investor, this event should be of interest to you. Multiple speakers explain how to acquire funding from Venture Capitalists and other investors.
AGENDA:
6:00 PM- 6:30 PM Registration and Networking and Life Sciences Speed Dating (Refreshments Served)
6:30 PM – 6:45 PM Dr. Jeffrey Hausfeld–Welcoming Remarks and Society of Physician Entrepreneurs Introduction
6:45 PM – 8:00 PM “Show Me the Money!” presentation followed by Q & A
back to top 
 
In partnership with BHI, the SBIR Resource Center(R) is offering a daylong seminar on How To Win SBIR Awards(SM) – at all federal agencies – with a primary emphasis on the National Institutes of Health (NIH). This event incorporates ALL of the necessary strategic planning, persuasive proposal writing, project planning/management and Federal project cost-accounting strategies to make your application successful. Walk away with a deep understanding of what it takes to win and an ability to customize SBIR/STTR win strategies for specific projects. Get equipped to evoke positive responses in SBIR/STTR proposal evaluators (very different from all other programs).
WHEN: Tuesday, 5 March 2013, 8:30am – 6:00pm (45 min. lunch break)
WHERE: Rockville Economic Development, Inc.95 Monroe Street – Rockville, MD 20850
(short walk from Rockville Metro Sta.)
301-315-8096 (location phone)
REGISTRATION: or call 410-315-8101 OR email the SBIR Center; Class limited to 18-20 participants
TUITION: $325 ($375 after February 25) — satisfaction assured or tuition is returned
DISCOUNT: BioHealth Innovation may underwrite $175 of the tuition for select biohealth companies through its Commercial Relevance Program.
To inquire about the discount, Contact Ethan Byler
back to top 

In this newsletter we proudly present the new Chairman of the Advisory Board: Richard Bendis.
With his expertise on innovation strategy The Technopolicy Network intends to strengthen
its position as the global leading network on Science Based Regional Development and Science Based Incubation. In his article he will give you several insights in the opportunities that lie ahead of us.
This is the time to pay tribute to the achievements of our founding Chairman, Prof. Roger Stough. With his advice and support Prof. Stough made The Technopolicy Network to what it now is. In his article he looks back on the growth of The Technopolicy Network over the past decade.
back to top 
 The next event in Tech Transfer Speaker Series will be taking place on February 13, 2013 in the William E. Hanna, Jr. Innovation Center (9700 Great Seneca Highway Rockville, Maryland 20850). The next event in Tech Transfer Speaker Series will be taking place on February 13, 2013 in the William E. Hanna, Jr. Innovation Center (9700 Great Seneca Highway Rockville, Maryland 20850).
TEDCO has undergone a number of changes over the past two years. These changes have resulted in a variety of new programs and changes to old programs. The talk will provide an overview of TEDCO’s new programs, including its affinity funds, and where TEDCO is headed as a funding organization.
Speaker:
Stephen Auvil is the senior vice president for technology transfer and commercialization at the Maryland Technology Development Corporation (TEDCO). In this role, he is responsible for overseeing TEDCO’s funding programs.
back to top 
Vaxin Inc., a clinical stage vaccine development company today announced the appointment of David Brake Ph.D. to its Board of Directors.
“Vaxin has a long history of product development in animal health. It will be great to have someone with David’s expertise in this area joining our Board,” said David J. Drutz, MD, Vaxin’s Chairman. “Vaxin has significant ongoing collaborations with Drs. Henry Baker and Nancy Cox at Auburn University in the development of a vaccine to sterilize dogs and cats and with Dr. Haroldo Toro also at Auburn University in the development of influenza and other vaccines for poultry. We now have someone to help provide valuable product development and business insights on these initiatives.”
back to top 

Patrick O’Shea, Vice President for Research at University of Maryland, urges Congress to protect research students and ensure america’s future by stoping the sequester.
back to top 

The University of Maryland, College Park (UMCP) was recently named as the 5th “Best Value in Public Colleges” by Kiplinger, trailing only the College of William and Mary, the University of Florida, the University of Virginia and the University of North Carolina at Chapel Hill.
Maryland was ranked 5th for in-state students and 10th for out-of-state.
The annual Kiplinger study bases its ranking on a combination of financial factors, including total cost-per-year and cost after need-based aid for in-state students, total cost-per-year and cost after need-based aid for out-of-state students and average debt at graduation. Kiplinger also factors in the schools’ admission rates and four-year-graduation rates.
back to top 

GlaxoSmithKline (GSK), the British pharmaceutical company, reported lackluster fourth quarter earnings for 2012 this morning, with a 3.5% drop in revenue. But the company’s performance would have been much worse if it hadn’t successfully avoided a looming threat that every brand-name pharmaceutical maker faces from time to time: the end of a patent on a blockbuster drug.
GSK’s Advair inhaler (called Seretide in most of Europe and India)—used to treat asthma and chronic obstructive pulmonary disease—lost its patent at the end of 2010. Ordinarily, a cheaper, generic version of a patented drug comes out shortly after the patent expires, and the generic quickly eats away at the marketshare and revenue of its branded progenitor. But Advair still brings $8 bln in sales to GSK, making it the third highest grossing drug worldwide. The only other off-patent pharmaceutical in the top ten is Lipitor, used for treating high cholesterol, which earned its maker, Pfizer, less than half as much in 2012 as it did in 2011, the year its patent expired (in spite of Pfizer’s unprecedented campaign to keep Lipitor a top-seller by strategically slashing prices).
back to top 

Julie Lenzer Kirk, Executive Director of the Howard County Economic Development Authority’s Maryland Center for Entrepreneurship and Co-Chair of Startup Maryland, will brief White House officials during an event intended to celebrate entrepreneurship and the Startup America Partnership. The briefing, by invitation only, will be held at 3 p.m., February 5 at the White House.
The Startup Maryland team was invited to share how their efforts have developed over the past year, as well as highlight the group’s themes and areas of concentration for the future. As one of the most active state-based regions over the past year, Startup Maryland has attracted more than 500 startup participants in the eight months since officially launching. In addition to Kirk, other Maryland participants include Startup Maryland Co-Chair and CEO of kloudtrack®, Mike Binko; Brian Murphy, founder/CEO of Smith Island Baking Co.; David Troy, CEO of 410Labs, Inc; and Johnny Shockley, Cofounder of Hooper’s Island Oyster Aquaculture Co.
back to top 
There’s a special place in NIH’s heart for SBIR research to develop drugs, medical devices and other products that require FDA approval. For these capital intensive products where time horizons for market entry are long, many NIH institutes offer extra millions and extra years of SBIR grant support after Phase II ends.
For most institutes, SBIR Phase IIB Competing Renewal grants are the vehicle for giving extra money. At NCI and NHLBI, Bridge grants do the same thing.
back to top 

A University of Maryland, College Park fundraising campaign that began in 2006 has reached its goal of $1 billion.
It was the largest fundraising drive ever undertaken by a public institution in the Washington and Baltimore region and the largest campaign of any public university in Maryland.
The school said it raised $1.008 billion from nearly 130 million individuals, companies and foundations, including 125,000 alumni.
back to top 

After its outstanding success in Boston in 2012, AdvaMed 2013: The MedTech Conference is back in our nation’s capital, Washington, DC. AdvaMed 2013 is the leading MedTech Conference in North America, bringing more than 1,000 companies together in a uniquely multifaceted environment for business development, capital formation, innovative technology showcasing, world-class educational opportunities and networking. This must-attend event for the MedTech industry will be held September 23 – 25 at the Walter E. Washington Convention Center. Please visit www.advamed2013.com for more information on the MedTech Conference.
back to top 

We all know that venture capitalists help entrepreneurs create and grow great companies. Those great companies create jobs and improve our standard of living. Yet what many don’t realize is that the traditional venture industry is consolidating.
Washington area private firms, such as New Enterprise Associates, Grotech, New Atlantic Ventures and Novak Biddle, that raise money from third-party investors are becoming fewer and farther between, with just over 500 such firms in the U.S. last year. Yet, our country’s most promising start-ups continue to get funded in part because of the rise of corporate venture capital.
back to top 

Startup Maryland (www.startupmd.org), a state-wide initiative FOR Entrepreneurs…BY Entrepreneurs, today announced a partnership with CoFoundersLab (www.cofounderslab.com) to provide the Maryland entrepreneur community with a free way to find a co-founder/business partner. To meet this need Startup Maryland and CoFoundersLab combined efforts and brands to develop a TeamFinder portal – which is also unveiled today as a new Resource at: http://startupmd.org/buildmyteam/
The branded TeamFinder portal is the first component of Startup Maryland’s Connection initiative. Connection joins Celebration, Coaching and Capital as four primary Areas of Concentration for Startup Maryland throughout 2013 and beyond. These four guiding initiatives were officially announced last week at a White House reception during which Startup Maryland highlighted past successes and future plans for Presidential advisors and officials from several government agencies.
back to top 

US venture capital (VC) funding in the life sciences sector, which includes the Biotechnology and Medical Device industries, dropped 14 percent in dollars and 7 percent in deals during 2012 according to a new PwC US report, “Double-digit dip” that includes data from the MoneyTree™ Report from PricewaterhouseCoopers LLP and the National Venture Capital Association based on data provided by Thomson Reuters. Venture capitalists invested a total of $6.6 billion in 779 Life Sciences deals during the year, compared with $7.7 billion in 836 deals during 2011. The number of Life Sciences companies receiving VC funding for the first time reached the lowest level since 1995 with only 135 companies receiving funding in 2012.
Compared to the prior quarter, Life Sciences venture funding rose 11 percent in Q4 2012 to $1.9 billion. Deal volume also increased, rising 12 percent to 187 deals compared to the prior quarter.
back to top 

Congressman Michael Honda (D., Calif.), who has been representing Silicon Valley in the U.S. House of Representatives for the past 12 years, recently submitted a bill asking for Congress to create and fund a new office at the U.S. Food and Drug Administration, which would be called the Office of Wireless Health.
The office would be tasked with regulating the growing number of mobile, wireless health gadgets and applications, which have been proliferating wildly since the start of the smartphone craze.
back to top 

There are more market research reports, survey results and industry metrics related to mobile and digital health floating around these days than in years past: Our recently published State of the Industry Q4/2012 Year in Review report included a summary of 16 different metric-loaded reports that published during the last three months of the year alone. That means results from one or more digital health surveys published each week leading up to the end of 2012.
While far from perfect, these market numbers help shape our perception of what’s really going on in the market at large. Rightly or wrongly, even small surveys can have this effect.
back to top 

Clinical stage biopharmaceutical company Catabasis Pharmaceuticicals Inc. has pulled in an $8.7 million round of funding, according to federal documents.
This is the not the first investment round for the Cambridge, Mass-based company which is focused on the development of treatments for metabolic and inflammatory diseases. In 2010, the company closed a $48 million Series A financing backed by SV Life Sciences, Clarus Ventures, MedImmune Ventures and Advanced Technology Ventures. In December 2011, the company received an $8 million Series A extension.
back to top 

As hospitals work to boost their efficiency and the quality of care they deliver, many are finding they need new ways to go about finding and creating solutions to their post-Affordable Care Act challenges. They’re also looking for new revenue streams.
With that in mind, one California health system put up $40 million to seed an independent, for-profit company that will help commercialize ideas that come from physicians and staff members while also feeding them promising new technologies and concepts from the outside.
back to top 
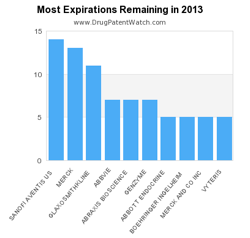
This infographic from DrugPatentWatch shows that Sanofi Aventis, Merck, and GlaxoSmithKline face the most patent expirations this year. Perhaps not surprisingly, GlaxoSmithKline and Sanofi Aventis are also among the firms with the most drug patents.
back to top 

Blue Button Plus (Automated and Interoperable Blue Button) can provide a technology path for startups and innovators to build new products and services to help Americans with their health. But beyond, technology startups and small businesses have to think about practical matters like funding. What are ways that the federal government is trying to help health startups, particularly health tech startups, develop and commercialize businesses?
The Small Business Innovation Research (SBIR) program is a federally-funded program encourages domestic small businesses to engage in Federal Research/Research and Development (R/R&D) that has the potential for commercialization. Through a competitive awards-based program, SBIR enables small businesses to explore their technological potential and provides the incentive to profit from its commercialization. By including qualified small businesses in the nation’s R&D arena, high-tech innovation is stimulated and the United States gains entrepreneurial spirit as it meets its specific research and development needs.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

February 13
Johns Hopkins University, Montgomery County Campus, Building III

February 16
Montgomery College

March 5
Rockville Economic Development, Inc. – Rockville, MD

March 20-22
Washington Convention Center, Washington, D.C.
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
POSITION DESCRIPTION – Entrepreneur-in-Residence
 The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets. The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets.
back to top 

In this newsletter we proudly present the new Chairman of the Advisory Board: Richard Bendis.
With his expertise on innovation strategy The Technopolicy Network intends to strengthen
its position as the global leading network on Science Based Regional Development and Science Based Incubation. In his article he will give you several insights in the opportunities that lie ahead of us.
This is the time to pay tribute to the achievements of our founding Chairman, Prof. Roger Stough. With his advice and support Prof. Stough made The Technopolicy Network to what it now is. In his article he looks back on the growth of The Technopolicy Network over the past decade.
back to top 

Jim Gates, physics professor and string theorist at the University of Maryland, is best known outside academia for his ability to explain the super-cerebral world of theoretical physics to scientific dummies.
In one oft-viewed PBS video, Gates endeavors to define string theory in 30 seconds, asking: What’s left after splitting an atom 35 times? “We have no instruments to measure that, and so people like me have been working on a piece of mathematics called string theory and superstring theory to answer that question. We think there are filaments there.”
back to top 

Two more venture capital firms have been selected to receive money for investing in early-stage businesses through the state’s InvestMaryland program.
New Atlantic Ventures in Reston will receive $8 million and Kinetic Ventures in Chevy Chase will receive $5 million through the program, which is run by the Maryland Venture Fund Authority. The $84 million InvestMaryland program will give two-thirds of its money to venture capital firms to invest in early-stage companies in Maryland and the Venture Fund Authority will invest the rest itself.
back to top 

The UMD $75K Startup Challenge is an intensive business model competition for students, staff, faculty, postdocs and recent alumni at the University of Maryland, College Park, and the University of Maryland, Baltimore to leverage their talent and ideas to create tomorrow’s leading companies.
INNOVATE
Take your research or great idea and start a company! Enter the UMD $75K Startup Challenge by February 22, 2013 with just an executive summary and three-minute video pitch.
Interested initial entrants can take advantage of the free, open entrepreneur office hours offered by Mtech for advice in preparing your submission.
back to top 

Qiagen NV reported earnings per share (EPS) of 16 cents in the fourth quarter of 2012, significantly up from the break-even EPS in the year-ago period. After adjusting for certain one-time items (other than stock-based compensation), adjusted EPS were 32 cents in the quarter, beating the Zacks Consensus Estimate by 2 cents and up 3 cents from the prior-year quarter. For fiscal 2012, the adjusted EPS came in at $1.00, in line with the Zacks Consensus Estimate and up 8.7% from fiscal 2011. T
Net sales in the quarter stood at $346.5 million, up 4% year over year (same at constant exchange rates or CER). Additionally, it surpassed the Zacks Consensus Estimate by $17.5 million. AmniSure (acquired in May 2012) made a 2% contribution to growth at CER. Also, excluding the impact of the year-ago product tender, organic growth was 4% at CER. The year-over-year improvement in sales was primarily on the back of strong performances by the company’s molecular diagnostics and applied testing customer classes.
back to top 

GlaxoSmithKline (GSK), the world’s fourth largest drug maker, has entered into an equal joint venture agreement with Biological-E Ltd., a vaccines company based in Hyderabad, recently. The report has unveiled that the two companies are giving a shot to a six-in-one vaccine.
The companies would conduct a deep research as they are aiming at developing such a combination paediatric vaccine that protects children in developing nations like India. It is being hoped that the combination vaccine could fight not only polio, but other infectious diseases as well.
back to top 

Further substantiating the school’s title as one of the most forward-thinking, cutting edge institutions the nation has to offer, the University of Maryland (UMD) has announced the launch of their first ever Academy for Innovation and Entrepreneurship today.
Due to launch in Fall 2013, the new Academy will instill a sense a culture of innovation and entrepreneurship into all areas of curriculum and colleges with the addition of classes, workshops and real world affairs that will allow students to truly grasp the meaning and need for creativity in the workplace.
back to top 

State officials are seeking some changes in the $84 million InvestMaryland program, including allowing the Department of Business and Economic Development to acquire a greater ownership interest when investing in a venture firm.
Current law prohibits DBED from acquiring an ownership interest of more than 25 percent in a business in which it invests. Legislation filed by Sen. Edward J. Kasemeyer (D-Dist. 12) of Columbia on behalf of DBED would allow the state to acquire a larger interest if the investment is in a venture or private equity firm.
back to top 

Silicon Valley venture capital giant New Enterprise Associates, known as NEA, is likely to open a Boston area office within the next six months. That’s according to General Partner David Mott, leader of the venture firm’s health care investing and former CEO of drug maker Medimmune, which was bought by AstraZeneca plc (NYSE: AZN) in 2007.
Mott is based in Washington D.C. but says he spends so much of his time in Boston, that it only makes sense to put out a shingle here.
back to top 
Portfolio optimization and strategic site selection are crucial for success in the industry’s new reality.
In the new reality for life sciences companies – one where the product development formula of the past no longer applies, where extensive M&A activity is needed to fill pipelines and mitigate risk, and where an increasing amount of attention and opportunity lie in emerging markets – prudent measures and strategic solutions are critical to succeed. Yet with all this change and uncertainty comes an immeasurable amount of opportunity.
back to top 

Consistently, Maryland stands at or near the top of national rankings for basic research and development. This will come as no surprise to many who do business in the state, whether it’s in government contracting, defense, health care, banking and finance, or in any one of the dozens of other key sectors of the knowledge economy as defined by the Silicon Valley model. But its much lower position in entrepreneurial activity—#33, according to a recent report by the Kauffman Foundation—is prompting the Merrick School of Business at the University of Baltimore to ramp up its efforts to provide first-rate preparation for the state’s future leaders of tech-oriented businesses, whether new or established in the marketplace.
Debuting this fall, the M.S. in Innovation Management and Technology Commercialization program is intended for working students who plan to transition from the laboratory to organizational management. It integrates technological, market and organizational issues into the core of the program. Students with science-and technology-based degrees can enhance their career potential, moving into management through the program’s four themes:
back to top 

The Baltimore area is a hub of intellectual and technological capital, but a new report says the city ranks behind the national curve when it comes to patenting this research.
The Baltimore region ranks 116th out of 358 metro areas across the country when it comes to per capita patent applications, according to a report released Friday by the Brookings Institution. And while the number of patents granted per year nationally has increased by roughly 50 percent, the number of patents granted in the Baltimore area has remained relatively the same since 1980, according to the report.
back to top 
Drug-resistant bacteria are a growing problem at hospitals across the country. The bacteria, such as Staphylococcus and Clostridium difficile, are difficult to prevent and impossible to treat.
“The problem is expanding, and it’s going up and up and up,” explains Dr. Trish Perl of Johns Hopkins Hospital in Baltimore. “We’re running out of antibiotics to treat, and so the challenge is can we prevent?”
back to top 

A New Jersey foundation that funds research coming out of the state’s medical school has agreed to replenish its investment arm with $5 million to pump into biotechnology startups at the pre-seed stage — one of the most difficult stages for companies to get funding.
The New Jersey Health Foundation’s Foundation Venture Capital Group in New Brunswick, New Jersey invests up to $500,000 in pre-seed stage companies spinning out of the University of Medicine and Dentistry of New Jersey. It is poised to close its 10th investment deal from the fund initially set up in 2006.
back to top 

Health IT venture capital funding totaled $1.2 billion in 2012, according to a report by communications and consulting firm Mercom Capital Group, Healthcare IT News reports.
The amount is more than 200% higher than 2011’s total of $480 million (Miliard, Healthcare IT News, 1/29).
According to the report, 163 health IT venture capital funding deals occurred in 2012, compared with 49 deals in 2011 and 22 deals in 2010 (Mercom report, January 2013).
back to top 

Surgeons have released pictures of the incredible moment an Iraq veteran who lost all four limbs in a roadside bomb blast had a double-arm transplant.
Brendan Marrocco, who was injured in the explosion almost four years ago, said he’s looking forward to driving and swimming after undergoing the operation.
‘I just want to get the most out of these arms, and just as goals come up, knock them down and take it absolutely as far as I can,’ Marrocco said yesterday.
back to top 
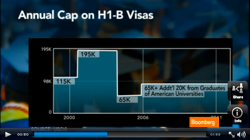
Just in case the momentum for comprehensive immigration reform falters, business groups have a back-up plan when it comes to high-skilled workers.
The Immigration Innovation Act, introduced in the Senate today, would raise the annual cap on H-1B visas from 65,000 to 115,000, and allow for additional visas if this cap is reached within a few months. Businesses in need of highly skilled workers use the H-1B visa program to fill these positions with foreigners.
back to top 

As we begin our 2013 search for the country’s most innovative entrepreneurs, we hope you’ll share the vision and nominate someone in your community, or even yourself, to be named among the next generation of business leaders. Ernst & Young’s Entrepreneur Of The Year is considered the world’s most prestigious business award with a lifelong network of gravity-defying entrepreneurs.
Each spring, the business community comes together to celebrate regional semifinalists, finalists and winners. These honorees not only create and build market-leading businesses, but also help take the standard of excellence to new heights, transform the face of industry, create jobs and contribute to the vibrancy of communities.
Applications will be accepted until March 8, 2013.
Click here to access the online application site or download the nomination brochure.
back to top 

Two leading biotechnology companies are competing to be the first to implement cheaper, faster processes for producing drugs inside living cells, making it easier to manufacture human proteins, antibodies, and other medications.
The new approaches will be “disruptively different” says Robert Bradway, the CEO of Amgen, one of the companies pursuing a manufacturing breakthrough. Today’s systems for producing drugs in bacterial or animal cells and then isolating them are hugely expensive and can take months. With more efficient processes in place, companies could swiftly increase production of drugs in high demand, and they could produce medicines for rare diseases more cost-effectively as well.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

February 5
Johns Hopkins University Montgomery County Campus

February 13
William E. Hanna, Jr. Innovation Center

February 13
Johns Hopkins University, Montgomery County Campus, Building III

February 16
Montgomery College

March 5
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|

Scott Carmer (BHI Board Chair), Jerry Parrott, and Rich Bendis (BHI CEO)
On January 24th, 2013 BioHealth Innovation, Inc. (BHI) recognized Jerry Parrott, formerly with Human Genome Sciences, for his contributions as a BHI Founding Board member. He served on the board from 2011 to 2013 and will continue to remain active on BHI’s Commercial Relevance Advisory Board (CRAB). We thank Jerry for his service and look forward to a long lasting relationship.
back to top 
POSITION DESCRIPTION – Entrepreneur-in-Residence
 The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets. The Entrepreneur-in-Residence (EIR) will lead in the evaluation of early-stage technologies, provide a strategic plan for start-up companies, advise BHI on opportunities for new ventures, and lead the commercial strategy for mature assets. The EIR influences the BHI organization by strategically managing and providing information, intelligence and insights that drive critical business decisions. The EIR will oversee primary and secondary research and will provide strategic recommendations and insights on the direction of potential assets.
back to top 

Are you interested in or know anybody that is interested in working at the forefront of e-Health? Our partner company Symcat have developed a mobile platform to aid people in identifying their symptoms while matching their data to thousands of other patient records.
Symcat is looking to fill the following positions:
Data Scientist
Marketing Director
Mobile Developer
Ruby Developer
To apply click here: http://symcat.theresumator.com/apply
back to top 
Director, Office of Translational Alliances and Coordination
Application Deadline Jan 28th, 2013

The National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) in Bethesda, Maryland is seeking a dynamic, innovative and accomplished biomedical/biotechnology executive with demonstrated scientific and entrepreneurial expertise to provide strategic vision and leadership for the Office of Translational Alliances and Coordination (OTAC). The OTAC is charged with developing, implementing and leading translational research programs that create recognizable commercial value for discoveries and innovations during their gestational stages and facilitating their ultimate translation into new diagnostics, devices, therapeutics and tools. The Office is also charged with identifying emerging areas of translational opportunities, serving as a focal point for extramural researchers for information on NHLBI-wide small business technology development opportunities.
back to top 

NHLBI Funding and Research Opportunities
The following funding opportunities from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Maryland is looking to build on the success of a biotechnology tax credit to bolster another industry here — cyber security.
Gov. Martin O’Malley proposed in his fiscal 2014 budget a new cyber security tax credit that would set aside $3 million to encourage cyber security companies to expand or set up shop in Maryland.
back to top 

New York Mayor Michael R. Bloomberg has pledged a $350 million gift to Johns Hopkins University to support interdisciplinary research and student financial aid, a commitment that will push his lifetime donations to his alma mater to more than $1 billion, the university announced Saturday.
Hopkins officials said they believe that Bloomberg will become the first person to reach the $1 billion level of giving to a single U.S. institution of higher education — an assertion that’s hard to verify because many donors give anonymously to universities. It is also difficult to compare the dollar value of modern donations to those in earlier eras.
back to top 

Venture capital firm SR One has launched “OneStart,” its first business plan competition, in association with Oxbridge Biotech Roundtable and Stevenage Bioscience Catalyst. OneStart is designed to inspire and encourage its participants to become the next generation of European bio- entrepreneurs. The £100,000 winner-takes-all prize will be the largest of its kind in the world. Uniquely, OneStart will actively connect individuals from a range of institutions and backgrounds.
OneStart is open to aspiring life science entrepreneurs age 35 and younger who are studying or working in Europe. The competition will be run in stages and cover four tracks: drug discovery, medical devices, diagnostics and health information technology. The competition will team up those who have developed a technology or idea, such as academics, with individuals who have complementary backgrounds and skills, e.g. business school students, or young professionals from pharma or biotech.
back to top 

Emergent BioSolutions Inc., a pharmaceutical company, has initiated the Phase II clinical trial for NuThrax or AV7909, with the dosing of the first subject.
NuThrax, a next generation vaccine being developed as part of Emergent’s anthrax franchise, consists of Anthrax Vaccine Adsorbed in combination with a novel immune stimulatory adjuvant, CPG 7909.
back to top 

Johns Hopkins University Whiting School of Engineering is collaborating with universities around the country on a project to create robots that work more efficiently with people. The National Science Foundation has funded the four-year, $3.5-million human-robot interaction research project, part of the National Robotics Initiative, a federal effort.
“In the world of robotics, there are two natural extremes: the completely autonomous robot and the fully technically-operated robot,” says Gregory Hager, chair of the computer science department at the engineering school.
back to top 
In 1953, Cambridge researchers Watson and Crick published a paper describing the interweaving ‘double helix’ DNA structure – the chemical code for all life.
Now, in the year of that scientific landmark’s 60th Anniversary, Cambridge researchers have published a paper proving that four-stranded ‘quadruple helix’ DNA structures – known as G-quadruplexes – also exist within the human genome. They form in regions of DNA that are rich in the building block guanine, usually abbreviated to ‘G’.
back to top 
District-based  Acceleprise, a business accelerator program for companies that sell technology or services to other companies or organizations, is expanding in an effort to serve more start-ups and raise its national profile. Acceleprise, a business accelerator program for companies that sell technology or services to other companies or organizations, is expanding in an effort to serve more start-ups and raise its national profile.
Started last spring, the program is the brainchild of managing partner Sean Glass and partners Allen Gannett and Collin Gutman. In addition to a $30,000 investment, participating start-ups spend several months honing business models and products at the Acceleprise office.
back to top 

A £50m venture capital fund focusing on early-stage life science and health technology companies has been launched in Scotland.
Rock Spring Ventures said it had already secured commitments worth more than £25m from a range of investors.
Backers include the universities of Glasgow, Edinburgh and Aberdeen.
back to top 
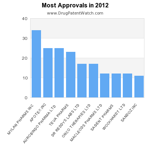
You might be surprised to find out which companies lead pharmaceutical industry drug approvals. This infographic from DrugPatentWatch shows that Mylan, Aurobindo, and Apotex led 2012 drug approvals. What some readers may find surprising is the relative absence of branded pharmaceutical firms from this list.
back to top 

It may just be early adopter tech types who log every step they take or calorie they burn using Fitbits, Nike Fuelbands, and other devices, but that hardly means they’re the only ones who track their health.
About 7 in 10 American adults told the Pew Internet & American Life Project that they track a health indicator like weight, diet, exercise or a symptom. But despite growing buzz around the “quantified self” movement and the explosion of gadgets and apps that help people measure and analyze everything from their activity and sleep patterns to blood glucose levels and other vital signs, just a small slice of health trackers rely on high tech devices.
back to top 
|
|
|


 |
In This Issue
|
| |
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

January 29
Institute for Bioscience and Biotechnology Research
January 31
Loews Annapolis Hotel, Annapolis, MD 21401

February 5
Johns Hopkins University Montgomery County Campus

February 13
William E. Hanna, Jr. Innovation Center

February 16
Montgomery College
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:
 |
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|

Rich Bendis (BHI) and Paul Vulto (MIMETAS) signing the MOU agreement.
MIMETAS is a Dutch microfluidics company focusing on high-throughput organ-on-a-chip systems for predictive toxicology testing, efficacy screening and personalized therapy.
BHI will support MIMETAS by assisting in establishing a MIMETAS US-based subsidiary, aiding in the application for US- and Maryland-based grants (e.g. SBIR, TEDCO), and identifying potential collaborators and partnerships.
This partnership is important in further expanding Maryland’s connections internationally and expanding Maryland’s biohealth sector.
back to top 

Peter Greenleaf is stepping down down as president of Gaithersburg-based biotechnology giant MedImmune, according to a company spokesman, to take the helm of parent company AstraZeneca’s Latin America business.
He will be replaced by Bahija Jallal, who currently serves as MedImmune’s executive vice president of research and development. Jallal joined the company in 2006 as vice president of translational sciences.
back to top 

Amid the exhaustive meet-and-greet opportunities available at the 2013 JP Morgan conference, which took place in San Francisco earlier this week, we met with Jens Eckstein, who joined SR One as its latest president slightly more than a year ago. SR One, as most IV Blog readers know, is the corporate venture arm of GlaxoSmithKline, and one of the oldest corporate venture funds in the industry. In an era in which such funds are assuming an ever-more important role in early-stage funding of innovative biotech companies, SROne’s priorities and strategic direction should be of great interest.
GSK has set broad parameters for SR One, with few restrictions; the firm invests with an eye on “the future of pharma in general,” not GSK, Eckstein says. GSK has never bought an SR One investment, and the firm steps aside if it sells one of its portfolio companies. He adds that the fund’s priority is early-stage innovation, with innovation defined broadly as “anything that changes the way medicine is done today.” That said, as SR One’s interests move “earlier and earlier” up the value chain, the evergreen funding provided by the corporate parent eliminates funding cycles and gives the venture firm a huge advantage over independent competitors.
back to top 

GlaxoSmithKline is looking to fatten its drug pipeline through another venture capital investment with a $50 million commitment to a California fund.
The drug giant on Wednesday said it would invest in the $250 million Sanderling Biotech Venture Fund. The fund will be managed out of San Mateo, Calif.
The commitment is one of several made by GSK (NYS: GSK) to several funds focused on helping emerging and early-stage companies advancing potential drug candidates.
back to top 

The Baltimore-area ranks fourth on a new Forbes list of the hottest tech markets in the country.
The report, conducted by Praxis Strategy Group, made its calculations based on STEM (science, technology, engineering, and mathematics) employment from 2001 to 2012 in the country’s 51 largest metropolitan areas.
The report says Baltimore’s STEM employment has grown 17.9 percent since 2001. In the last two years, STEM employment rose by 3.9 percent.
back to top 
AstraZeneca has been talking about the importance of expanding its network of collaborations at the JPMorgan healthcare conference in San Francisco.
Blogging from the meeting, Shaun Grady, vice president of strategic partnering and business development at the Anglo-Swedish drugmaker, noted that AstraZeneca and its MedImmune unit have a team of 60 “scouting for opportunities that fit our therapeutic and commercial focus”. He added that the firm has scheduled a record number of meetings at the event, approximately 320 in total.
back to top 

The Tech Council of Maryland called on legislators Monday to triple the funding for the state’s research and development tax credit and double the scope of its popular biotech tax credit, among other measures.
The Tech Council of Maryland places the expansion of the R&D credit from $6 million to $18 million among its top priorities for the 2013 General Assembly session, which convenes in Annapolis on Wednesday. The measure failed to win approval last year despite passing the Senate. The Tech Council also wants to see that credit made available for companies that haven’t yet reached profitability.
back to top 

Baltimore City’s technology incubator, ETC (Emerging Technology Center), announced today that six companies have been selected to participate in the 2013 AccelerateBaltimore™ program –a 50% increase from the first AccelerateBaltimore™-. The program received over 120 applications from all over the world. The three month accelerator will kick off on February 4, 2013.
“The quality of the applications and the number of participants was amazing. It made the job of choosing the final participants very difficult,” says Deb Tillett, President of ETC. “We had the help of some professionals from the tech community and it was a challenging but fun process. Congratulations and thanks to all I can’t wait to get started.”
back to top 

Emergent BioSolutions, Inc., recently signed a license agreement for the manufacture and sale of VaxInnate Corporation’s recombinant influenza vaccine.
The deal is considered to be a step in the right direction towards preparing the United States for the event of a major influenza pandemic. The licensing agreement grants Emergent BioSolutions, Inc., the right to manufacture and sell VaxInnate’s pandemic flu vaccine in the United States. VaxInnate, in turn, will receive payments and royalties associated with vaccine’s production and sale.
back to top 

Bicoastal venture titan New Enterprise Associates ranked as the most active VC firm in the country last year, according to investment research firm CB Insights.
NEA, which has major offices in Chevy Chase, beat out Kleiner Perkins Caufield & Byers, Google Ventures, Andreesen Horowitz and other top VCs, according to the report, which attributes NEA’s exuberance to its shiny new $2.6 billion fund. Major NEA deals last year include Sonatype Inc., Desire2Learn, 10Gen and Lithium Technologies.
back to top 

Companies in Maryland, Virginia and D.C. raised a paltry $94.9 million in venture funding in the last three months of 2012, according to the PricewaterhouseCoopers LLP MoneyTree report, marking one of the shabbiest quarters in years.
The region hasn’t seen this weak of a fundraising total since the $89.7 million raised in the first quarter of 2009, according to historical MoneyTree data. Excluding that period, the end of 2012 would have ranked as the worst quarter since the first quarter of 1997.
back to top 

Gov. Martin O’Malley released a $37 billion spending plan Wednesday that for the first time in recent years contains no drastic cuts or proposed tax increases.
Amid a stronger economy, O’Malley also proposed to boost the pay for state workers, expand tax credits for some high-tech industries and set aside more money to clean up the Chesapeake Bay.
“These have been challenging years to say the least,” O’Malley said.
back to top 

British healthcare firm GlaxoSmithKline has filed for US regulatory approval of its new type 2 diabetes drug albiglutide, which belongs to the same group of injectable GLP-1 receptor agonists as Byetta, Bydureon and Victoza .
GSK announced on Monday that it had submitted the once-weekly medication to the US Food and Drug Administration (FDA) for approval and confirmed that it also plans to seek European Union regulatory approval for the new product in 2013.
back to top 

MedImmune executive Bahija Jallal will be the new chief of the Gaithersburg biotech under a leadership shuffle by parent company AstraZeneca, a spokesman confirmed Wednesday. Current MedImmune President Peter Greenleaf will lead AstraZeneca’s Latin American business.
Jallal, who joined MedImmune in 2006 as a vice president of translational sciences, has also been promoted to AstraZeneca’s senior executive team, according to an announcement on Tuesday. That announcement, however, left unclear exactly how her role at MedImmune would change, and how that change would affect Greenleaf.
back to top 

Using cervical fluid obtained during routine Pap tests, scientists at the Johns Hopkins Kimmel Cancer Center have developed a test to detect ovarian and endometrial cancers. In a pilot study, the “PapGene” test, which relies on genomic sequencing of cancer-specific mutations, accurately detected all 24 (100 percent) endometrial cancers and nine of 22 (41 percent) ovarian cancers. Results of the experiments are published in the January 9 issue of the journal, Science Translational Medicine.
The investigators note that larger scale studies are needed before clinical implementation can begin, but they believe the test has the potential to pioneer genomic-based cancer screening tests.
back to top 

The buzz around biotechnology investing that could be felt this year at the J.P. Morgan Healthcare Conference could reach the beleaguered medical-device sector as well.
But it won’t be a spate of IPOs—and the headlines they generate–that re-ignites interest in device companies, several investors said.
“What we are really hoping to see in 2013 are great new products approved, and put on the market,” said Ryan Drant, a general partner at New Enterprise Associates and a board member at the Medical Device Manufacturers Association.
back to top 

If I’ve said it once, I’ve said it one bajillion times. If you’re going to start a business accelerator, don’t clone Y Combinator or TechStars. Find a niche. With the glut of new accelerators today, the most successful are building vertical-specific approaches that bring together seed capital with meaningful industry partnerships to create real business (and learning) opportunities for their startups. Lately, digital health has been leading the way in this regard, as Rock Health, Healthbox, New York Digital Health Accelerator, DreamIt Health and Startup Health are all beginning to blossom.
One of the veterans (a relative term) of this space is the NYC-based Blueprint Health, an accelerator that got its start in 2011 and launched its first batch in January of last year. Today, the accelerator is announcing its third batch of startups as part of its Winter Program, which kicks off on Monday.
back to top 

Another day, another class of digital health startups. That’s how it feels sometimes, with so many accelerators working with dozens of new companies each year. While there are plenty of “me-too” startups entering crowded markets, accelerators are still managing to find some fresh and interesting gems.
Blueprint Health’s winter program kicks off this week with the addition of Verizon, Humana and Aetna as partners, as TechCrunch reports. It’s also brought on mentors from powerhouses like the Cleveland Clinic, Optum, Kleiner Perkins Caufield & Byers and Weight Watchers.
back to top 
There might be a lot of griping about how tight the venture capital world is when it comes to funding medical devices, but reality seems to be spinning a different yarn altogether.
A new funding report from CB Insights, a venture capital database, describes the medical device industry as the “sector darling” of VCs who invested in healthcare in the fourth quarter of 2012. In fact medical device deals comprised 42 percent of the overall number of healthcare deals done in the fourth quarter of last year. By comparison biotech deals stood at 15 percent, drug development at 13 percent and pharmaceuticals at 10 percent.
back to top 

The Federal Communications Commission will make $400 million available annually to healthcare providers to expand the development of broadband telehealth networks from a pilot to a permanent program. The pilot program has supported 50 provider healthcare networks in 38 states.
The telehealth networks will link urban medical centers to rural clinics or offer instant access to electronic health records (EHRs). The agency will begin accepting applications for the grants in late summer, according to the Jan. 7 announcement by FCC Chairman Julius Genachowski.
back to top 

Venture capitalists invested $1.4 billion in digital health companies in 2012, according to a report from Rock Health, FierceHealthIT reports (Gold, FierceHealthIT, 1/8).
The figure represents a 45% increase from the $986 million invested in 2011. In addition, the total number of venture capital deals in the digital health sector increased by 56% between 2011 and 2012.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

January 22
Bethesda Marriott
February 19
Johns Hopkins Carey Business School – The Legg Mason Tower

March 20-22
Washington Convention Center, Washington, D.C.
|
Newsletter designed and distributed by:
 |
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
| | | | | |