
 Biotechnology company developing early liver diseases detection technology
Biotechnology company developing early liver diseases detection technology
COLUMBIA, Md., (August 19, 2025) — TEDCO, Maryland’s economic engine for technology companies, announced a recent $250,000 Pre-Seed Builder Fund investment in AGED Diagnostics. Housed under TEDCO’s Social Impact Funds, the Pre-Seed Builder Funds provide investment and support to Maryland-based technology companies led by founders from economically underserved backgrounds.
“TEDCO’s funding continues to support us in furthering our research and development for early liver disease detection,” said Rachel Zayas, CEO of AGED Diagnostics. “With this follow-on investment, we hope to move closer to our mission of providing access to early detection and intervention care for those with liver disease.”
AGED Diagnostics, based in Rockville, Md., is a biotechnology company developing an early detection and intervention solution for liver disease. Using genomic innovation, the company is working to create a more accurate blood test that could keep patients in a monitoring program, helping to reduce financial burdens and improve patient outcomes.
“AGED Diagnostics seeks to tackle both the health and financial aspects of liver disease through their detection technology,” said Jean-Luc Park, deputy chief investment officer & senior director of TEDCO’s Social Impact Funds. “At TEDCO, we work to support innovative companies like this who are working towards impactful solutions; through the Pre-Seed Builder Fund, we are honored to offer more than just funding in support for our portfolio companies.”
TEDCO’s Social Impact Funds are just one of the many investment opportunities available to qualifying technology-focused startups and small businesses across Maryland.
“As technologies continue to evolve, it is always promising to see innovation in biotechnology seek to support more efficient and affordable healthcare,” said TEDCO CEO, Troy LeMaile-Stovall. “Being ranked as a top state for health care, Maryland has a fertile ecosystem for entrepreneurs and companies looking to advance in the medical landscape.”
For more information, visit our page at www.tedcomd.com/funding.
About TEDCO
TEDCO, the Maryland Technology Development Corporation, enhances economic empowerment growth through the fostering of an inclusive entrepreneurial innovation ecosystem. TEDCO identifies, invests in, and helps grow technology and life science-based companies in Maryland. Learn more at www.tedcomd.com.
###
Media Contact
Tammi Thomas, Chief Development & Marketing Officer, TEDCO, tthomas@tedcomd.com
Rachael Kalinyak, Associate Director, Marketing & Communications, TEDCO, rkalinyak@tedcomd.com

 The Baltimore Innovation Initiative awards a total of more than $645,000 to 14 awardees
The Baltimore Innovation Initiative awards a total of more than $645,000 to 14 awardees
COLUMBIA, Md., (August 18, 2025) — TEDCO, Maryland’s economic engine for technology companies, announced the first round of the Baltimore Innovation Initiative (BII) Pilot Program awardees, marking an important milestone for the organization. The BII Pilot Program falls under the Maryland Innovation Initiative (MII), fueling innovation and economic impact in the Baltimore region.
“The first round of BII awardees exemplify the innovation present throughout the Baltimore-Columbia-Towson Metropolitan Statistical Area,” said Abi Kulshreshtha, executive director, MII. “These awards will advance innovative technologies from seven colleges and universities, contributing to Maryland’s growing innovation ecosystem.”
The BII was created to support an equitable innovation and entrepreneurial ecosystem within higher education institutions in the Baltimore-Columbia-Towson Metropolitan Statistical Area (MSA). As part of Maryland’s matching contribution toward the Baltimore Tech Hub, the BII seeks to advance technology toward commercialization of a product or service and bolster support for entrepreneurs developing technology-based ventures.
“We received a number of excellent candidates – choosing the 14 awardees was a difficult process, but we feel these awardees are best positioned to utilize the funding provided to progress towards commercialization,” said Jalaycia Lewis, BII program manager.
The Technology Advancement Grant offers applicants support to commercialize a new or existing technology at an eligible institution while the Entrepreneurship and Commercialization Programming and Infrastructure Grant supports the creation or enhancement of entrepreneurship programs and commercialization infrastructure for technology-based ventures.
The eight Technology Advancement awardees include:
- Selena Shirkin,Fetal Therapy Technologies: Designing a novel port system for microsurgery; Johns Hopkins University
- Rebecca Greene,Semiautonomous Humanistic Algorithm for Reduced Effort and Enhanced Dexterity (SHARE2D) Control; Johns Hopkins University
- Anika Mistry,DevMinds: adaptive social-emotional learning to support neurodiverse kids beyond therapy sessions; Johns Hopkins University
- Oriol Cuxart,A breakthrough neuromodulation solution for Chronic Rhinosinusitis patients; Johns Hopkins University
- Michael Tangrea, advancing precision medicine through commercialization of the micropurification technology; Loyola University Maryland
- Kevin Tu, developing a secure and integrated fume hood management platform to reduce energy waste in research laboratories; University of Maryland, Baltimore
- Andres Londoño,DegreeMap: A smart degree planning platform for Maryland higher education; Towson University
- Debbie Sahlin, ACCESS-DP Advancing Coordinated Care and Emerging Smart Solutions for Disabled Populations; University of Maryland, Baltimore
The six Entrepreneurship Commercialization, Programming and Infrastructure Awardees include:
- Ian Ryu,AI Venture Studio Pilot; Johns Hopkins University
- Wendy Bolger, Support for the Baltipreneurs Accelerator; Loyola University Maryland
- Ryan Hoover, Launching the MICA Biodesign Program to Support and Diversify the Regional Bioeconomy; Maryland Institute College of Art
- Henry Mortimer,UBalt AI-Enabled Business Incubator; University of Baltimore
- Kenneth Wong,Center for Climate and Health Entrepreneurship; University of Maryland, Baltimore
- Jamie Gurganus,Embedding Entrepreneurial Mindset in Academia: Launching the Entrepreneurial Learning Lab at UMBC; University of Maryland, Baltimore County
“Innovative entrepreneurs thrive in Maryland with the support of programs like BII,” said TEDCO CEO, Troy LeMaile-Stovall. “As the number one state for minority-owned businesses, Maryland is moving the needle forward. By embracing diversity, we continue to see growth in a merit-based ecosystem that will not only bring the state to the forefront of innovation but will allow for sustainability.”
The MII was established as a collaboration between the State of Maryland and five academic research institutions, and leverages each institution’s strengths while promoting the commercialization of research conducted there. Since its inception, the program has invested more than $59 million in promising research commercialization, supported nearly 200 companies and generated almost 400 jobs. The success of the MII led to several pilot programs that expanded MII’s reach to two more comprehensive universities in addition to the creation of BII.
To learn more about the MII and BII programs, eligibility and application details, visit tedcomd.com/funding/maryland-innovation-initiative.
About TEDCO
TEDCO, the Maryland Technology Development Corporation, enhances economic empowerment growth through the fostering of an inclusive entrepreneurial innovation ecosystem. TEDCO identifies, invests in, and helps grow technology and life science-based companies in Maryland. Learn more at www.tedcomd.com.
###
Media Contact
Tammi Thomas, Chief Development & Marketing Officer, TEDCO, tthomas@tedcomd.com
Rachael Kalinyak, Associate Director, Marketing & Communications, TEDCO, rkalinyak@tedcomd.com
In this episode of BioTalk with Rich Bendis, Stefanie Trop, Ph.D., Director of Life Sciences at the Maryland Department of Commerce joins the conversation to discuss Maryland’s position as a leading life sciences hub. Stefanie shares how the Department of Commerce supports company attraction and growth through programs like Global Gateway, and how it’s amplifying the state’s recent #3 biopharma hub ranking. The discussion also explores Maryland’s unique ecosystem, including the work of the Governor’s Life Sciences Advisory Board, the state’s leadership in computational health, and the importance of industry collaboration during BioHealth Capital Region Week.
Listen now on your favorite podcast platform:
Apple: https://apple.co/4oHp9Po
Spotify: https://bit.ly/4fEIooK
iHeart: https://ihr.fm/4fGY3UL
Amazon Podcasts: https://amzn.to/47Awvya
YouTube Podcasts: https://bit.ly/3V7rJAI
Editing and post-production work for this episode was provided by The Podcast Consultant.
Dr. Stefanie Trop brings a unique blend of hands-on scientific expertise and high-level strategic insight. With a Ph.D. in Molecular Microbiology and Immunology, she has worked at the ground level in immunology, clinical trials, and vaccine development, while also driving growth through scientific partnerships and business development. Her deep understanding of the science and passion for problem solving guides her ability to build effective collaborations that advance both innovation and commercial success.
At the Maryland Department of Commerce, Stefanie works to connect Maryland’s life sciences companies with national and global resources, expanding the industry’s impact at home and globally. In current and prior roles, she bridges communication gaps between scientific and business teams, ensuring that customer needs translate into innovative products and business wins.

 GERMANTOWN, Md, Aug. 15, 2025 /PRNewswire/ — Precigen, Inc. (Nasdaq: PGEN), a biopharmaceutical company specializing in the advancement of innovative precision medicines to improve the lives of patients, today announced that the US Food and Drug Administration (FDA) has approved PAPZIMEOS™ (zopapogene imadenovec-drba) for the treatment of adults with recurrent respiratory papillomatosis (RRP). PAPZIMEOS is the first and only FDA-approved therapy for the treatment of adults with RRP. Precigen completed submission of the rolling Biologics License Application (BLA) in December 2024 under an accelerated approval pathway; however, the FDA has granted PAPZIMEOS full approval, which does not require a confirmatory clinical trial. PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy designed to express a fusion antigen comprising selected regions of human papillomavirus (HPV) types 6 and 11 proteins—the root cause of RRP. PAPZIMEOS is delivered via four subcutaneous injections over a 12-week interval.
GERMANTOWN, Md, Aug. 15, 2025 /PRNewswire/ — Precigen, Inc. (Nasdaq: PGEN), a biopharmaceutical company specializing in the advancement of innovative precision medicines to improve the lives of patients, today announced that the US Food and Drug Administration (FDA) has approved PAPZIMEOS™ (zopapogene imadenovec-drba) for the treatment of adults with recurrent respiratory papillomatosis (RRP). PAPZIMEOS is the first and only FDA-approved therapy for the treatment of adults with RRP. Precigen completed submission of the rolling Biologics License Application (BLA) in December 2024 under an accelerated approval pathway; however, the FDA has granted PAPZIMEOS full approval, which does not require a confirmatory clinical trial. PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy designed to express a fusion antigen comprising selected regions of human papillomavirus (HPV) types 6 and 11 proteins—the root cause of RRP. PAPZIMEOS is delivered via four subcutaneous injections over a 12-week interval.
RRP is a rare, debilitating, and potentially life-threatening disease of the upper and lower respiratory tract caused by chronic HPV 6 or HPV 11 infection. RRP can lead to severe voice disturbance, a compromised airway, and recurrent post-obstructive pneumonias. Management of RRP has primarily consisted of repeated surgeries, which do not address the root cause of the disease and can be associated with significant morbidity as well as significant patient and health system burden.
“For more than a century, since RRP was first recognized as a distinct disease, patients have had to rely on repeated surgeries to manage this relentless condition. Today marks a historic turning point. With the landmark FDA approval of PAPZIMEOS and broad label, all adult RRP patients are now eligible for access to the first and only approved therapy that targets the root cause of the disease,” said Helen Sabzevari, PhD, President and CEO of Precigen. “This milestone affirms the power of our AdenoVerse platform and the exceptional capabilities of our team to rapidly advance a wholly novel therapy from discovery to approval considerably faster than industry benchmarks. We are profoundly grateful to the NIH clinicians, the FDA, and—most importantly—the patients and families who made this breakthrough possible. We look forward to swiftly delivering PAPZIMEOS to the RRP community and ushering in a new era of treatment that targets the underlying cause of the disease rather than just managing its symptoms.”
“This long-awaited FDA approval represents a momentous milestone for the RRP community,” said Kim McClellan, President of the Recurrent Respiratory Papillomatosis Foundation. “For the first time, adult patients with RRP have access to an FDA-approved therapy that offers the potential to reduce—or even eliminate—endless repeated surgeries. This breakthrough brings long-overdue hope to patients and families who have endured so much. We are deeply grateful to the teams at Precigen and the NIH, and above all, to the patients and caregivers whose courage, advocacy, and perseverance have made this historic moment possible.”
The approval is supported by data from the open-label, single-arm, pivotal study in adult patients with RRP:
- The pivotal study successfully met its primary safety and pre-specified primary efficacy endpoints.
- 51% (18 out of 35) of study patients achieved Complete Response, requiring no surgeries in the 12 months after treatment with PAPZIMEOS. These Complete Responses remained durable for over 12 months. Of the 18 patients with a Complete Response in the ongoing study, 15 patients evaluated at 24 months demonstrated continued Complete Response.
- PAPZIMEOS was well-tolerated with no dose-limiting toxicities and no treatment-related adverse events greater than Grade 2.
- PAPZIMEOS induced HPV 6/11-specific T cell responses in RRP study patients with a significantly greater expansion of peripheral HPV-specific T cells in responders compared with non-responders.
The pivotal study was led by lead investigators, Clint T. Allen, MD, and Scott M. Norberg, DO, at the National Institutes of Health. Pivotal data were presented at the 2024 American Society of Clinical Oncology (ASCO) annual meeting and published in The Lancet Respiratory Medicine.
Precigen will begin promoting PAPZIMEOS immediately and is committed to helping patients with RRP access the therapy. Precigen has established Papzimeos SUPPORT, a comprehensive patient support program offering personalized services, including insurance navigation, financial assistance, and ongoing access support, which can be accessed by calling 866-827-8180. Healthcare professionals interested in learning more about PAPZIMEOS or accessing provider support services are encouraged to visit www.PAPZIMEOS.com.
Conference Call
The Company will host a conference call on Monday, August 18 at 8:00 AM ET to provide additional details regarding the approval, including key aspects of the label and commercialization. Event details can be found on Precigen’s website in the Events & Presentations section at investors.precigen.com/events-presentations.
About RRP
RRP is a rare, debilitating, and potentially life-threatening disease of the upper and lower respiratory tract caused by chronic HPV 6 or HPV 11 infection. RRP can lead to severe voice disturbance, compromised airway, and recurrent post-obstructive pneumonias. Although rare, RRP has the potential for transformation to malignant cancer and can be fatal. Management of RRP has primarily consisted of repeated surgeries, which do not address the underlying cause of the disease and can be associated with significant morbidity as well as significant patient and health system burden. As the number of lifetime surgeries increases, the risk for irreversible iatrogenic laryngeal injury increases with each surgery, and patients may undergo hundreds of these surgeries over their lifetimes. RRP can impact patients’ work and social lives, financial stability, and mental health. Patients with RRP can experience substantial impacts to daily living with decreased quality of life and high health care utilization. Based on an internal analysis of claims data and electronic health records, there are approximately 27,000 adult RRP patients in the US.
About PAPZIMEOS™ (zopapogene imadenovec-drba), for subcutaneous injection only
PAPZIMEOS is the first and only FDA-approved therapy for the treatment of adults with RRP and the first and only approved therapy to address the root cause of RRP. PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy designed to express a fusion antigen comprising selected regions of human papillomavirus (HPV) types 6 and 11 proteins. PAPZIMEOS is designed to generate an immune response directed against HPV 6 and HPV 11 proteins in patients with RRP. Discovered and designed in Precigen’s labs using Precigen’s proprietary AdenoVerse therapeutic platform, PAPZIMEOS represents a new therapeutic paradigm for RRP.
Indication and Important Safety Information
What is PAPZIMEOS?
PAPZIMEOS is a type of immunotherapy used to treat a condition called recurrent respiratory papillomatosis (RRP) in adults.
What is the most important information I should know about PAPZIMEOS?
Some people may have a reaction to the shot. Signs and symptoms may include redness, pain, swelling, itching, or warmth where the shot was given. After your first treatment, your healthcare provider will watch you for at least 30 minutes to make sure you’re feeling okay.
Please contact your doctor immediately if you develop an infection, the reaction to your shot worsens, or you experience any of the below symptoms, which may indicate a systemic allergic reaction:
- Difficulty breathing
- Widespread rash
- Facial swelling
Thrombotic events (blood clots that block your blood vessels) may occur after your PAPZIMEOS shot. Please notify your doctor immediately if you have the following symptoms:
- Shortness of breath
- Chest pain
- Leg swelling
- Persistent abdominal pain
- Severe or persistent headaches
- Blurred vision
What should I know before taking PAPZIMEOS?
Before taking PAPZIMEOS, tell your healthcare provider about all of your medical conditions, including:
- If you are pregnant or plan to become pregnant because it is not known if PAPZIMEOS will harm the unborn baby.
- If you are breastfeeding or plan to breastfeed. It is unknown if PAPZIMEOS is present in breast milk, or how it affects the breastfeeding child or milk production. Talk to your healthcare provider about the best way to feed your baby during treatment with PAPZIMEOS.
What are the most common side effects of PAPZIMEOS?
The most common side effects include:
- Pain, redness, or swelling where the shot was given
- Feeling tired
- Chills
- Fever
- Muscle aches
- Nausea (feeling sick)
- Headache
- Increased heart rate
- Diarrhea
- Vomiting
- Sweating a lot
These are not all of the possible side effects of PAPZIMEOS. Call your healthcare provider for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088. You may also report side effects to Precigen, Inc. at 1-855-PGE-NRRP (1-855-743-6777).
Please see full Prescribing Information.
Precigen: Advancing Medicine with Precision®
Precigen (Nasdaq: PGEN) is a biopharmaceutical company specializing in the advancement of innovative precision medicines to address difficult-to-treat diseases with high unmet patient need. Precigen is dedicated to advancing scientific breakthroughs from proof-of-concept through commercialization. With a strong commitment to innovation, Precigen is developing a robust pipeline of differentiated therapies across its core therapeutic areas of immuno-oncology, autoimmune disorders, and infectious diseases. For more information about Precigen, visit www.precigen.com or follow us on LinkedIn or YouTube.
Trademarks
Precigen, PAPZIMEOS, AdenoVerse, and Advancing Medicine with Precision are trademarks of Precigen and/or its affiliates. Other names may be trademarks of their respective owners.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking” statements within the meaning of the safe harbor provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “anticipate,” “intend,” “plan,” “goal,” “seek,” “believe,” “project,” “estimate,” “expect,” “strategy,” “future,” “likely,” “may,” “should,” “will” and similar references to future periods. These statements are subject to numerous risks and uncertainties that could cause actual results to differ materially from what the Company expects. Examples of forward-looking statements include, among others, information relating to the Company’s business and business plans, the success of efforts to commercialize PAPZIMEOS™ (zopapogene imadenovec-drba) for the treatment of recurrent respiratory papillomatosis (RRP) in adults, the Company’s ability to successfully obtain foreign regulatory approvals for PAPZIMEOS, expectations about the safety and efficacy of PAPZIMEOS and the Company’s other product candidates, the timing of clinical trials and their results, the Company’s ability to commence clinical studies or complete ongoing clinical studies, and the ability of PAPZIMEOS to treat RRP. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in the Company’s most recent Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission.
Investor Contact:
Steven M. Harasym
Tel: +1 (202) 365-2563
investors@precigen.com
Media Contact:
Donelle M. Gregory
press@precigen.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-full-fda-approval-of-papzimeos-zopapogene-imadenovec-drba-the-first-and-only-approved-therapy-for-the-treatment-of-adults-with-recurrent-respiratory-papillomatosis-302530957.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/precigen-announces-full-fda-approval-of-papzimeos-zopapogene-imadenovec-drba-the-first-and-only-approved-therapy-for-the-treatment-of-adults-with-recurrent-respiratory-papillomatosis-302530957.html
SOURCE Precigen, Inc.

 by: Karuga Koinange – Brian Jamieson founded Diagnostics Biochips to unlock the inner workings of the brain.
by: Karuga Koinange – Brian Jamieson founded Diagnostics Biochips to unlock the inner workings of the brain.
Based in Glen Burnie, the medical equipment manufacturing company develops tiny sensors that can be implanted to study electrical brain activity. The goal? To help find cures for neurological diseases like Parkinson’s, epilepsy and others.
Diagnostic Biochips also offers a data analysis platform called DBCloud that tracks and organizes the vast neural activity captured by its sensors. Together, these tools give researchers a window into what Jamieson describes as the brain’s “matrix.”
“If you’re probing a network or a computer and you want to see the 1s and 0s that make up the basic operation of a microprocessor, it’s the same thing with your brain,” Jamieson said. “What is the basic underlying machine-level code of your brain? It’s not a perfect metaphor, but it has some of the same characteristics in that you have all these basic elements that are connected.”
A loan that led to a new line of business
The State of Maryland’s venture capital arm, TEDCO, was one of the first to bet on Diagnostic Biochips, providing a seed investment that helped bring the company to life in 2013.
When the company first launched, it focused on building tools for in vivo applications, i.e., research with live animals. After a decade in this line of business, Jamieson had aspirations to scale up.
In 2023, he turned his attention to advanced stem cell models such as pluripotent stem cells (known as iPSCs) and 3D cultures called organoids. Brain organoid models are miniature, lab-grown versions of the human brain small enough to fit in a petri dish that replicate key properties of a developing, normal or diseased brain. Using these models, researchers can drastically boost the volume and efficiency of their data collection.
“Instead of doing individual surgeries on animals, now we’re scanning 96 brains at a time,” Jamieson said.
To support this pivot, Diagnostic Biochips started seeking funding for stem cell research. That search led Jamieson to apply for the Maryland Stem Cell Research Fund (MSCRF) in December 2024.
Click here to continue reading.
In this episode of BioTalk, Rich Bendis welcomes Dr. Stacey Adam, Vice President of Science Partnerships, Translational Science at the Foundation for the National Institutes of Health (FNIH), to discuss how public-private partnerships are advancing scientific innovation. Dr. Adam introduces the mission of FNIH and its unique role in bridging government, industry, and academia to accelerate biomedical progress. She highlights the Validation and Qualification Network (VQN), a new initiative working to overcome barriers to the adoption of New Approach Methodologies (NAMs) and explains how cross-sector collaboration is driving its early success. The conversation explores the long-term vision of the VQN, the global perspectives shaping its approach, and how it fits into the broader NIH Complement-ARIE initiative. Dr. Adam also reflects on the significance of being headquartered in the BioHealth Capital Region and how it supports the Foundation’s mission.
Listen now via your favorite podcast platform:
Apple – https://apple.co/3UngVhK
Spotify – https://bit.ly/411Mdym
YouTube Podcasts – https://bit.ly/4m3tY3Y
iHeart – https://ihr.fm/4locw9o
Amazon Podcast – https://amzn.to/3JaSO3l
Editing and post-production work for this episode was provided by The Podcast Consultant.
Guest Bio
Dr. Stacey Adam is Vice President of Science Partnerships at the Foundation for the NIH (FNIH), where she leads major public-private partnerships including the RECOVER-Treating Long COVID initiative, multiple Biomarkers Consortium projects, the Accelerating Medicines Partnerships (AMPs), and the Lung-MAP clinical trial. She also leads efforts to design new public-private partnerships focused on pediatric medical devices, cancer systems biology, and the advancement of New Approach Methodologies (NAMs) through the Validation and Qualification Network. Previously, Dr. Adam worked at Deloitte Consulting and completed a postdoctoral fellowship at Stanford University School of Medicine. She holds a PhD in Pharmacology with a Certificate in Mammalian Toxicology from Duke University.


For decades, the National Institutes of Health (NIH) has been the cornerstone of the United States’ biomedical research enterprise. But new policies introduced by the current administration, alongside a proposed 40% cut to the NIH’s FY 2026 budget, are sending shockwaves through the research community. The impacts are already visible, and deeply concerning for institutions, researchers, and innovative ecosystems across the country, including the BioHealth Capital Region (BHCR).
As first reported in Science by Jocelyn Kaiser, NIH has begun implementing a major shift in how it funds research grants: 50% of multiyear awards must now be funded in full, up front, rather than distributed year by year over the life of the grant. This policy was initiated without Congressional approval, though the 2026 budget proposal may expand this to a larger number of grants, with the percentage expected to rise to 100% by FY27. The impact of this is already reshaping funding outcomes for FY 2025.
 In practice, this means that instead of spreading the cost of a three-year grant across three annual appropriations, NIH must now allocate the full amount from the current year’s budget. As a result, far fewer grants can be awarded.
In practice, this means that instead of spreading the cost of a three-year grant across three annual appropriations, NIH must now allocate the full amount from the current year’s budget. As a result, far fewer grants can be awarded.
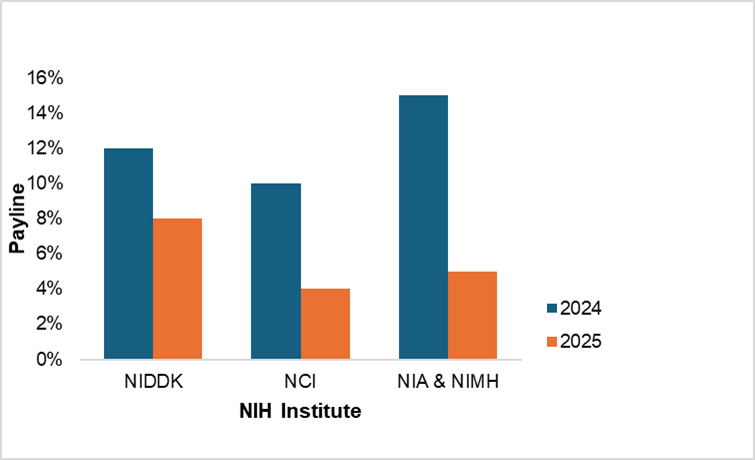
According to Kaiser’s reporting in Science, the National Cancer Institute’s (NCI) grant payline has dropped from 10% to 4%. A reduction so sharp means that many investigators will no longer consider applying. Other NIH institutes are facing similar cuts:
- The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) payline is projected to drop from 12% to 5–8%.
- The National Institute on Aging (NIA) and the National Institute of Mental Health (NIMH) are each expected to fund only one-quarter to one-third as many grants as last year, reducing paylines from 15% to 4–5%.
Adding to these concerns, the NIH is reported to be conducting additional assessments of grants that have already undergone and passed scientific peer review, introducing uncertainty into a system once governed by rigor, transparency, and merit.
Full article via Science:
https://www.science.org/content/article/odds-winning-nih-grants-plummet-new-funding-policy-and-spending-delays-bite
As public policy expert Don Moynihan writes in his Substack piece, this new multi-year funding policy was imposed not by NIH leadership, but by political appointees at the Department of Health and Human Services or the White House. NIH employees are not in favor of this requirement and have been working to mitigate its damage internally. However, the rapid pace of implementation, amid an already complex fiscal year, is compounding the disruption.
Moynihan notes that the percentage of awarded applications across NIH is expected to drop by a factor of 2 to 4, leading to widespread lab closures, layoffs, and stalled medical research. The article also warns that this policy may be used to free up future NIH budgets for politically driven initiatives that bypass traditional scientific vetting.
Full article via Substack:
https://donmoynihan.substack.com/p/alert-the-trump-administration-is
The BioHealth Capital Region is home to the NIH, the FDA, over 1,800 life sciences companies, and hundreds of academic and clinical research institutions. The region’s strength, and its #3 ranking for the third consecutive year in the GEN Top 10 U.S. Biopharma Clusters list, rests in part on sustained federal support for biomedical research and commercialization.
A significant disruption in NIH grantmaking could disproportionately affect this region, where many companies and academic centers rely on NIH funding to support R&D, build talent pipelines, and bring new innovations to market.
As a public-private innovation intermediary, BioHealth Innovation, Inc. (BHI) is working closely with startups, entrepreneurs, and researchers across the region to navigate the changing landscape. BHI’s support for non-dilutive funding strategy and commercialization services has never been more critical.
Despite these challenges, well-crafted grant applications may still succeed. BioHealth Innovation’s Manager of Client Engagement, Jon Nelson, points out: “We’re certainly in the midst of one of the most difficult funding environments that the BioHealth sector has seen in a long time. However, experienced grant writers will be able to employ carefully crafted aims, thoughtful research approaches, and strategic key phrasing to continue to secure the desperately needed funding.”
Congressional leaders from both parties have expressed concern about the administration’s actions, including the possibility of rescinding unspent NIH funds at the end of the fiscal year. But time is short: the federal fiscal year ends on September 30, and decisions made in the next few weeks will shape the future of U.S. biomedical research for years to come.
BHI will continue monitoring these developments and advocating for policies that preserve America’s leadership in biomedical innovation. We urge stakeholders across the region to stay informed, connect with their Congressional representatives, and elevate the voices of scientists, innovators, and patients who depend on stable, merit-based research funding.
Please contact Jon Nelson, Ph.D., Manager of Client Engagement,
JNelson@BioHealthInnovation.org, if your organization is looking for assistance in this space.
