![]()
Johns Hopkins Center for Health Security authors recommend dedicated attention to and investment in ‘potentially transformative’ technologies to complement traditional approaches to prevention, preparedness, and response
![]()
Johns Hopkins Center for Health Security authors recommend dedicated attention to and investment in ‘potentially transformative’ technologies to complement traditional approaches to prevention, preparedness, and response


The Center for Innovative Technology (CIT) announced today the Commonwealth Research Commercialization Fund (CRCF) Request for Proposals (RFP) for FY2019.
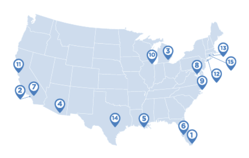

Some big cities could see shortages of cancer specialists in the coming decade, according to a new analysis by Doximity, a social network for more than a million physicians. Researchers at the company delved into the data on oncologists and found there’s an imminent wave of retiring physicians. The areas most likely to see shortages: Miami, Virginia Beach, and Tampa. But other cities could also see large portions of the cancer physician workforce retire relatively soon. In half of the 50 metropolitan areas surveyed, more than 20 percent of practicing oncologists are over age 65.


The local funding environment is mirroring the gangbusters national funding situation.


Valneva USA, the U.S. subsidiary of global vaccine biotech company Valneva SE, today announced U.S. Food and Drug Administration (FDA) approval of an accelerated dosing regimen for IXIARO® (Japanese Encephalitis Vaccine, Inactivated, Adsorbed), the only vaccine approved in the United States indicated for protection against disease caused by Japanese encephalitis (JE) virus.


BioHealth Innovation sponsors quarterly office hours in Montgomery County and at Johns Hopkins Fast Forward (Fast Forward hours are for JHU innovators only).
BioHealth Capital Region innovators not located at Fast Forward can register for SBIR office hours on the following dates.
To register for the open sessions, click here. (BHI also will be hosting a more general SBIR overview with office hours on January 29, 2019. Mark your calendar; more details to follow.)


If you and your team are located in the BioHealth Capital Region, BHI may prepay for experts to help you improve your SBIR and other non-dilutive funding applications for biohealth related projects. Your only obligation is that if you win the award, you reimburse BHI for the consulting fees (typically $1500 or $3000) and a minimal program management fee (ten % of the prepaid consulting fee i.e. $150 or $300).
For more information contact BHI.


Are you a start-up in Maryland, DC or Virginia seeking feedback on your biohealth business idea, pitch deck, or commercialization plan? business/commercialization plan, pitch deck, or idea?
Schedule your feedback session with BHI EIRs on one of the following dates:
(30 minute blocks of time beginning at 9:00 in the morning. If the morning slots are filled, more time will be allotted beginning at 1 p.m.)
Pre-registration is required; sign up here (“EIR resource”at BHI). For questions/more information, contact BHI.


The University of Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI) has been renewed for funding under a cooperative agreement grant from the U.S. Food and Drug Administration (FDA).
A collaborative partnership led by James Polli, PhD, the Shangraw/Noxell Endowed Chair in Industrial Pharmacy and Pharmaceutics at the University of Maryland School of Pharmacy (UMSOP), and William E. Bentley, PhD, the Robert E. Fischell Distinguished Chair of Engineering at the University of Maryland, College Park (UMCP), M-CERSI is one of only four FDA-funded CERSIs in the United States and the only CERSI to receive continuous funding from the FDA since it launched in 2011.


Led by Joel Marcus, Founder of Alexandria Real Estate Equities
October 9th at 4:20pm
One MedImmune Way, Gaithersburg, MD 20878
Networking Reception to follow
RSVP by 2 p.m. 10/8 to: amy@taylormadeexperience.com