

The chief executive will stay in his role, and on the board, until his successor is appointed or early next year, whichever comes first.


The chief executive will stay in his role, and on the board, until his successor is appointed or early next year, whichever comes first.
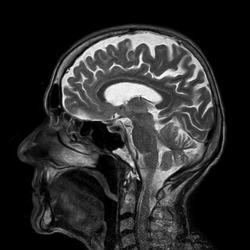

he man was 66 when he came to the hospital with a serious skin infection. He had a fever and low blood pressure, as well as a headache. His doctors gave him a brain scan just to be safe. They found a very small bulge in one of his cranial arteries, which probably had nothing to do with his headache or the infection.


American Gene Technologies (AGT), a leading gene and cell therapy company in the discovery and development of lentiviral vector based therapeutics, announces that the U.S. Food and Drug Administration (FDA) received orphan-drug designation #DRU-2018-6572 for the treatment of phenylketonuria (PKU) using its proprietary, lentiviral vector based technology. PKU is a debilitating inherited disease affecting 1 in 13,500 children born in the U.S. Mandatory screening identifies affected children who can be supported with a strict synthetic diet that does not cure disease. If successful, AGT’s lentiviral vector approach will provide a permanent cure, improving quality of life for more than 16,000 people living with PKU in the U.S. alone.


PathoVax, a Johns Hopkins–born startup developing a vaccine for human papillomavirus virus (HPV), raised $2.75 million in new funding, cofounder Joshua Wang said.


United Therapeutics will license, develop, and commercialize CollPlant Holdings’ recombinant human collagen (rhCollagen) and BioInk technology for 3D bioprinting of solid-organ scaffolds for human transplants, the companies said today, through a collaboration that could generate more than $44 million.
![]()
REGENXBIO Inc. (Nasdaq: RGNX), a leading clinical-stage biotechnology company seeking to improve lives through the curative potential of gene therapy based on its proprietary NAV® Technology Platform, today announced that Ultragenyx Pharmaceutical Inc. has exercised an option for an exclusive worldwide license to REGENXBIO’s NAV Vectors, including NAV AAV9, for the treatment of CDKL5 Deficiency Disorder (CDD).


Children benefit from little of the $156 billion annual U.S. market for medical devices, but a “Shark Tank”-inspired partnership between the University of Maryland and Children’s National Health System is looking to change that.


Jean Cui led the invention of Pfizer’s Xalkori. Now she has her own company focusing on drug-resistant cancers, and it has raised $147 million.

![]()
Investors sentiment decreased to 1.37 in 2018 Q2. Its down 0.26, from 1.63 in 2018Q1. It dived, as 22 investors sold GlycoMimetics, Inc. shares while 16 reduced holdings. 25 funds opened positions while 27 raised stakes. 45.91 million shares or 6.89% more from 42.95 million shares in 2018Q1 were reported.


Viela Bio heads to the 2018 American College of Rheumatology / Association of Rheumatology for Health Professionals (ACR/ARHP) Annual Meeting in Chicago, 19-24 October 2018, with a late-breaking poster presentation for a novel engineered CD40L antagonist, VIB4920.