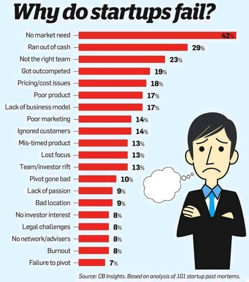
With its world-class institutions, talent and collaboration, now is the time for Maryland to take the next steps in becoming the nation’s premier science and tech-based innovation and entrepreneurial corridor.

Pocket-size ultrasound devices that cost 50 times less than the machines in hospitals (and connect to your phone). Virtual reality that speeds healing in rehab. Artificial intelligence that’s better than medical experts at spotting lung tumors. These are just some of the innovations now transforming medicine at a remarkable pace.

In March 2019, BioSpace wrote about how the BioForest Hotbed area was the fastest-growing life sciences market in the top 10 from 2014 to 2017. Although relatively small compared to the top areas such as California and Massachusetts, Washington state and Seattle are growing at faster than 17%. One of the life sciences’ heavy hitters in real estate development, Alexandria Real Estate Equities, is showing its increasing interest in the region.

NIH, Gates Foundation invest $200M to develop gene therapies for developing countries – MedCity News

Although touted as having potential to cure some of the most intractable of human illnesses, gene therapies also carry some eye-popping price tags that have threatened to limit their availability to wealthy countries. However, a new public-private partnership aims to make them available to people around the world, including in developing countries.


NEW YORK, Oct. 25, 2019 /PRNewswire/ — The Prix Galien USA Committee last night honored excellence in the biopharmaceutical and medical industry for research, development and innovation at its 13th annual Prix Galien Awards Gala, held at the American Museum of Natural History in New York City. In recognition of the development and discovery of products that improve the human condition, a committee of highly accomplished scientific leaders, inclusive of four Nobel Laureates, recognized winners in three categories: “Best Pharmaceutical Product,” “Best Biotechnology Product,” and “Best Medical Technology.”
Image: “Best Pharmaceutical Product:” GlaxoSmithKline, SHINGRIX


BALTIMORE, Md., Oct. 21, 2019 (GLOBE NEWSWIRE) — CoapTech LLC announced today that the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) within the National Institutes of Health (NIH) awarded the company a two-year, $1.2M R44 Small Business Innovation Research (SBIR) Phase II grant on August 29, 2019. This builds on a $225,000 Phase I SBIR R43 grant and a $200,000 Maryland Industrial Partnerships (MIPS) grant awarded to the company last year.
The new $1.2M grant will support a 40-patient clinical trial at the University of Maryland Medical Center (UMMC) evaluating safety and cost-effectiveness characteristics of a novel medical procedure known as percutaneous ultrasound gastrostomy (PUG), which is enabled by CoapTech’s PUMA-G device.


ROCKVILLE, Md., Oct. 18, 2019 /PRNewswire/ — American Gene Technologies (AGT) announced today the submission of an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for AGT’s lead HIV program, AGT103-T, which is potentially a single-dose, lentiviral vector-based gene therapy developed for the purpose of eliminating HIV from people infected with the disease.
Image: https://www.prnewswire.com


The General Assembly of Maryland has enacted Senate Bill 137 (the “General Corporate Legislation”), which makes several changes to the Maryland General Corporation Law (the “MGCL”) and the Maryland REIT Law (the “MRL”). The General Assembly has also enacted Senate Bill 136 to explicitly permit the use of distributed electronic networks or databases (sometimes referred to as “blockchain” technology) in corporate record keeping (the “Distributed Ledger Legislation”). Finally, the General Assembly enacted House Bill 1116 and the identical Senate Bill 911 regarding annual reporting on the composition of the boards of directors of certain Maryland corporations (the “Annual Report Legislation”). The new legislation was signed by Governor Hogan and became effective on October 1, 2019. Unless otherwise noted, all section references below are to the MGCL.