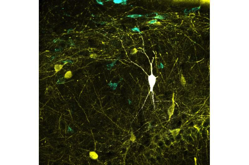

A study led by researchers from the Institute Cajal of Spanish Research Council (CSIC) in Madrid, Spain in collaboration with the Bioengineering Department of George Mason University in Virginia, U.S. has updated one of the world’s largest databases on neuronal types, Hippocampome.org.
The study, which is published in the journal PLOS Biology, represents the most comprehensive mapping performed to date between neural activity recoded in vivo and identified neuron types. This major breakthrough may enable biologically meaningful computer modeling of the full neuronal circuit of the hippocampus, a region of the brain involved in memory function.
Image: https://medicalxpress.com