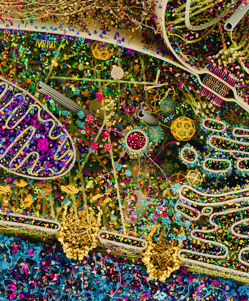

Most Detailed #Visualization of Human Cell Ever Created – this 3D model of a eukaryotic cell is created using X-ray, nuclear magnetic resonance (NMR), and cryo-electron microscopy datasets. It is an attempt to visualize the many pathways involved in cellular processes (i.e. signal transduction, protein synthesis, endocytosis, vesicular transport, cell-cell adhesion, apoptosis) as well as the great complexity & beauty of the cell’s molecular machinery.
Image: https://www.linkedin.com