
The first person to receive a transplanted heart from a genetically modified pig is doing well after the procedure last week in Baltimore, Maryland. Transplant surgeons hope the advance will enable them to give more people animal organs, but many ethical and technical hurdles remain.
“It’s been a long road to get to this point, and it’s very exciting we are at a point where a group was ready to try this,” says Megan Sykes, a surgeon and immunologist at Columbia University in New York City. “I think there’s going to be a lot of interesting things to be learned.”
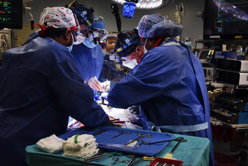
Image: Surgeons at the University of Maryland Medical Center transplanted a genetically altered pig heart into David Bennett.Credit: University of Maryland School of Medicine