
 In this episode of BioTalk, we welcome Rick Wieczorek, President and CEO of the NIH Federal Credit Union (NIHFCU), to discuss how credit unions like NIHFCU are uniquely positioned to support the biohealth and healthcare communities. With over 40 years of industry experience, Rick shares his journey from teller to CEO and reflects on NIHFCU’s 85-year history, its mission-driven approach to service, and the importance of tailoring financial solutions to meet the needs of life science professionals. He also explains the benefits of membership, the value of the NIHFCU “At Work” program for employers, and how the credit union’s “Banking with Heart” philosophy is making a difference across the BioHealth Capital Region.
In this episode of BioTalk, we welcome Rick Wieczorek, President and CEO of the NIH Federal Credit Union (NIHFCU), to discuss how credit unions like NIHFCU are uniquely positioned to support the biohealth and healthcare communities. With over 40 years of industry experience, Rick shares his journey from teller to CEO and reflects on NIHFCU’s 85-year history, its mission-driven approach to service, and the importance of tailoring financial solutions to meet the needs of life science professionals. He also explains the benefits of membership, the value of the NIHFCU “At Work” program for employers, and how the credit union’s “Banking with Heart” philosophy is making a difference across the BioHealth Capital Region.




 What does it take to establish Maryland as a powerhouse for biotech commercialization? In this episode of BioTalk with Rich Bendis, Dr. Deborah Hemingway, Founder and Managing Partner of Ecphora Capital, shares her insights on the current investment landscape in the BioHealth Capital Region. She discusses the strategic advantages that make Maryland uniquely positioned for success, the critical gaps that must be addressed, and how state and local governments can provide essential support through tax credits, grants, and economic incentives.
What does it take to establish Maryland as a powerhouse for biotech commercialization? In this episode of BioTalk with Rich Bendis, Dr. Deborah Hemingway, Founder and Managing Partner of Ecphora Capital, shares her insights on the current investment landscape in the BioHealth Capital Region. She discusses the strategic advantages that make Maryland uniquely positioned for success, the critical gaps that must be addressed, and how state and local governments can provide essential support through tax credits, grants, and economic incentives.
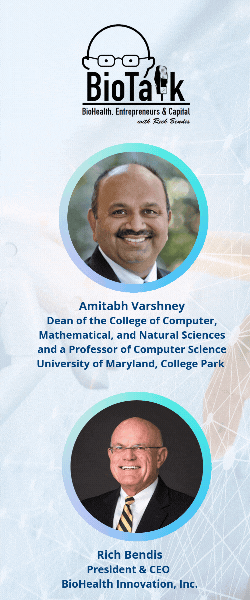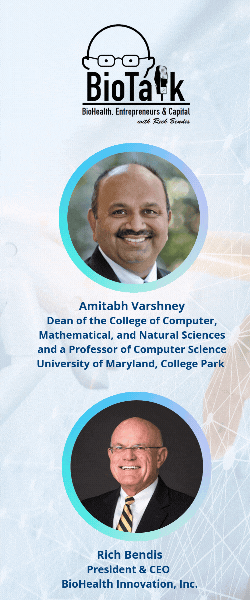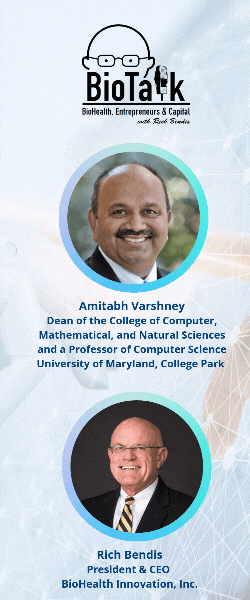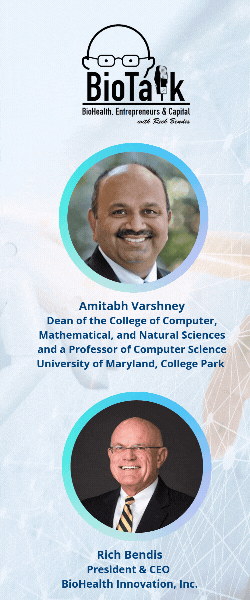 In this episode of BioTalk, host Rich Bendis is joined by Amitabh Varshney, Dean of the College of Computer, Mathematical, and Natural Sciences and a Professor of Computer Science at the University of Maryland, College Park. Together, they explore the groundbreaking advancements in artificial intelligence and health computing driven by the University of Maryland (UMD).
In this episode of BioTalk, host Rich Bendis is joined by Amitabh Varshney, Dean of the College of Computer, Mathematical, and Natural Sciences and a Professor of Computer Science at the University of Maryland, College Park. Together, they explore the groundbreaking advancements in artificial intelligence and health computing driven by the University of Maryland (UMD).
 In this episode of BioTalk with Rich Bendis, Dr. Kolaleh Eskandanian, Vice President and Chief Innovation Officer at Children’s National Hospital, discusses her work driving pediatric healthcare innovation. Dr. Eskandanian introduces the BARDA SPARK Accelerator, a groundbreaking initiative focused on advancing medical countermeasures for children, and explains how it aligns with Children’s National’s mission to lead in pediatric healthcare innovation.
In this episode of BioTalk with Rich Bendis, Dr. Kolaleh Eskandanian, Vice President and Chief Innovation Officer at Children’s National Hospital, discusses her work driving pediatric healthcare innovation. Dr. Eskandanian introduces the BARDA SPARK Accelerator, a groundbreaking initiative focused on advancing medical countermeasures for children, and explains how it aligns with Children’s National’s mission to lead in pediatric healthcare innovation.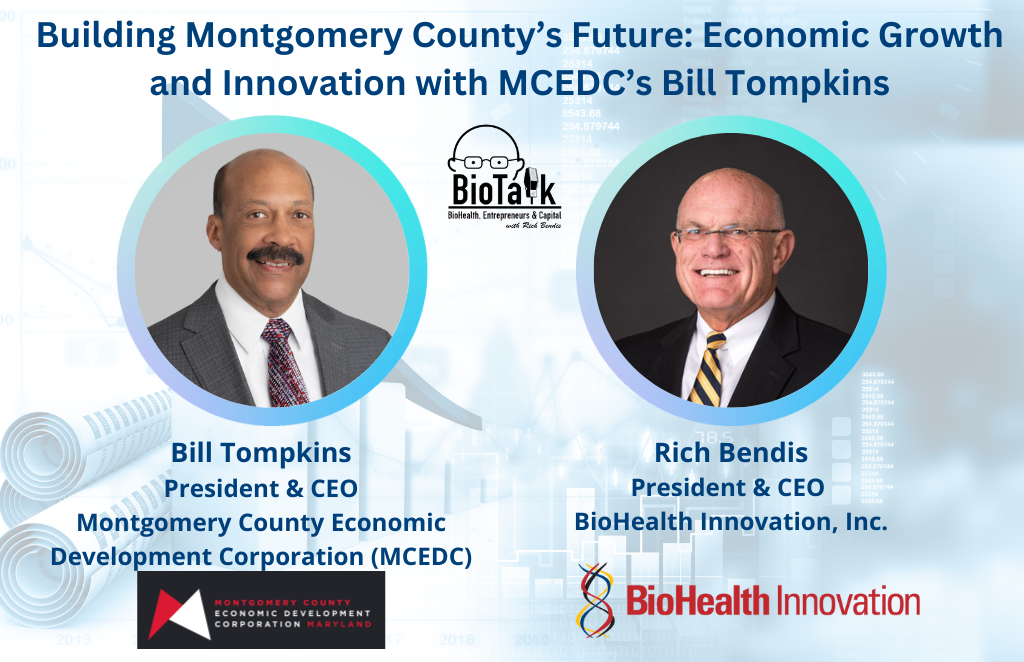
 In this episode of BioTalk, Bill Tompkins, President and CEO of the Montgomery County Economic Development Corporation (MCEDC), discusses the strategies driving Montgomery County’s position as a leading bioscience hub. Bill highlights MCEDC’s role in reinforcing the region’s standing as the third-largest bioscience cluster in the U.S. and shares insights into the new Institute for Health Computing. He also introduces two new venture funds—the Technology Innovation Fund and the Founders Fund—designed to accelerate innovation and support local entrepreneurs.
In this episode of BioTalk, Bill Tompkins, President and CEO of the Montgomery County Economic Development Corporation (MCEDC), discusses the strategies driving Montgomery County’s position as a leading bioscience hub. Bill highlights MCEDC’s role in reinforcing the region’s standing as the third-largest bioscience cluster in the U.S. and shares insights into the new Institute for Health Computing. He also introduces two new venture funds—the Technology Innovation Fund and the Founders Fund—designed to accelerate innovation and support local entrepreneurs.
 In this episode of BioTalk, host Rich Bendis welcomes Ric Hughen, CEO of Linshom Medical, and Talia Feldman, Software Engineer at Linshom Medical, to discuss their groundbreaking advancements in respiratory monitoring technology. As the main winners of the 2024 Crab Trap Competition at the 10th Annual BioHealth Capital Region Week, Ric and Talia share Linshom’s mission to improve patient safety through their innovative and accessible respiratory monitoring devices.
In this episode of BioTalk, host Rich Bendis welcomes Ric Hughen, CEO of Linshom Medical, and Talia Feldman, Software Engineer at Linshom Medical, to discuss their groundbreaking advancements in respiratory monitoring technology. As the main winners of the 2024 Crab Trap Competition at the 10th Annual BioHealth Capital Region Week, Ric and Talia share Linshom’s mission to improve patient safety through their innovative and accessible respiratory monitoring devices.
 In this episode of BioTalk with Rich Bendis, Dr. Eric Edwards, Co-Founder and CEO of MedPhlow, shares how his innovative company is reshaping emergency medicine through cutting-edge drug delivery systems. Dr. Edwards discusses his journey from co-founding Kaléo, where he helped develop life-saving products like AUVI-Q, to leading MedPhlow, a subsidiary of Phlow Corp. Based in Richmond, VA, MedPhlow is dedicated to addressing critical challenges in essential medicine delivery, with its first product focused on improving outcomes during sudden cardiac arrest.
In this episode of BioTalk with Rich Bendis, Dr. Eric Edwards, Co-Founder and CEO of MedPhlow, shares how his innovative company is reshaping emergency medicine through cutting-edge drug delivery systems. Dr. Edwards discusses his journey from co-founding Kaléo, where he helped develop life-saving products like AUVI-Q, to leading MedPhlow, a subsidiary of Phlow Corp. Based in Richmond, VA, MedPhlow is dedicated to addressing critical challenges in essential medicine delivery, with its first product focused on improving outcomes during sudden cardiac arrest.
 Join us for an insightful episode of BioTalk as Rich Bendis speaks with Eric Mayton, CEO of Cerillo, one of the recent winners at the Crab Trap competition during the 10th Annual BioHealth Capital Region Week. Cerillo, based in Charlottesville, VA, is making waves in microbiome research and biological product development with its innovative tools designed to tackle variability challenges and enhance data collection efficiency.
Join us for an insightful episode of BioTalk as Rich Bendis speaks with Eric Mayton, CEO of Cerillo, one of the recent winners at the Crab Trap competition during the 10th Annual BioHealth Capital Region Week. Cerillo, based in Charlottesville, VA, is making waves in microbiome research and biological product development with its innovative tools designed to tackle variability challenges and enhance data collection efficiency.