|
|
|
 BHI CEO Rich Bendis recently served as an expert panelist contributing to a special report from the “White House Lab-to-Market Inter-Agency Summit.” Panelists examined the U.S. federal government’s investment in drug R&D and the resulting private sector commercialization. According to the Summit report: BHI CEO Rich Bendis recently served as an expert panelist contributing to a special report from the “White House Lab-to-Market Inter-Agency Summit.” Panelists examined the U.S. federal government’s investment in drug R&D and the resulting private sector commercialization. According to the Summit report:
Federal research has done exceedingly well at accomplishing its original intent, which is to increase human knowledge, meet mission needs, and undertake high-risk research of long-term importance to the U.S. economy that is beyond the reach of the private sector. But commercialization of resulting discoveries from agency research has largely been an after-thought, despite clear Congressional and Presidential intent expressed through a series of legislative mandates and Executive Orders. While research to meet agency missions is critical, the members of the Panel believe that if the U.S. is to remain globally competitive in the 21st century, it must accelerate the translation of federally-funded R&D into commercial outcomes that create economic and public value, thus maximizing the return on the public dollars invested.
Read more at:
Increasing the Impact of Federally-Funded R&D
back to top 
by Joseph P. Allen and Diane Palmintera
 On May 20, 2013, the White House Lab-To-Market Inter-Agency Summit was held in Washington, D.C. The Summit was organized by the White House Office of Science and Technology Policy and the National Institutes of Health’s Heart, Lung, and Blood Institute. The purpose of the meeting was extraordinary: asking national experts outside of the federal agency system to recommend ways to increase the return-on-investment for the $140 billion annual taxpayer expenditure on federally-funded research and development. On May 20, 2013, the White House Lab-To-Market Inter-Agency Summit was held in Washington, D.C. The Summit was organized by the White House Office of Science and Technology Policy and the National Institutes of Health’s Heart, Lung, and Blood Institute. The purpose of the meeting was extraordinary: asking national experts outside of the federal agency system to recommend ways to increase the return-on-investment for the $140 billion annual taxpayer expenditure on federally-funded research and development.
The format for the meeting also was unusual. Research agencies nominated 20 national experts experienced in various phases of technology commercialization to participate in the Summit. The Administration placed no preconditions or limitations on the expert Panel and asked it to focus on “transformative” ideas. We were privileged to be asked to serve as the Summit’s co-chairs.
The Administration requested that the Panel address several overarching questions:
- How can agencies better align themselves to more effectively promote the commercial development of their research;
- How can effective metrics for various stages of these efforts be developed; and
- How can we better leverage multi-agency resources to enhance the public’s return-on-investment through the commercialization of more federally-funded technologies?
back to top 

Topic: “EIRs, SBIRs, and more with BioHealth Innovation, Inc.”
Presenters:
- Richard Bendis, President and CEO
- Ethan Byler, Director, Innovation Programs
- Todd Chappell, Entrepreneur-in-Residence, NIH-OTT
- Dr. Ken Malone, Entrepreneur-in-Residence, UMD Ventures
- Ram Aiyar, Entrepreneur-in-Residence, NHLBI
BioHealth Innovation, Inc. (BHI) is a regional innovation intermediary that accelerates and facilitates technology transfer and commercialization of market-relevant research in federal labs, universities, and biohealth companies in the Region. It is a private-public partnership that connects the Region’s innovation assets to provide integrated technical knowledge, financial means, and entrepreneurial/managerial expertise to turn promise into prosperity for the region while advancing human health.
BHI’s Entrepreneur-in-Residence (EIR) program is designed to be an active partner with research institutions to source, fund, and grow high-potential, early-stage products through project-focused companies. The entrepreneurs in the program support the formation of new companies based upon innovative discoveries in the areas of drugs, vaccines, therapeutics, diagnostics, and medical devices from the intramural research programs at the NIH and Food and Drug Administration (FDA), as well as from universities and businesses.
BHI’s Commercial Relevance Program (CRP) offers biohealth companies support in preparing applications for federal funding inclusive of SBIRs, STTRs, and other federal government awards. Companies submit their federal funding concepts and receive pre-proposal feedback to help troubleshoot and strengthen your application. Further support from professional consultants and service providers is available to assist in improving your application.
BHI recently published the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide serves as a compendium of resources to biohealth innovators and entrepreneurs working to start and grow new companies and technologies in the region.
back to top 

The INNo program trains research scientists in the entrepreneurial skills needed to bring technology inventions and services to the healthcare market.
Participants in the INNo program learn to:
- Identify and evaluate the commercial potential of intellectual property
- Understand the business fundamentals related to technology start-ups
- Create a value proposition and business concept for a new product, platform, or service
- Articulate investment opportunities persuasively to potential investors and partners
- Develop a network of resources in the Maryland entrepreneurial community
back to top 
 The RESI conference is poised to be one of the more unique events in the life sciences space this coming fall. This full-day investor partnering conference is groundbreaking in that it is focused on redefining the investor landscape in early stage life sciences. As all of us in the industry are aware, the life science investor landscape has changed; venture capital has left a void and there is a plethora of new entities entering the space with capital to allocate. The RESI conference is poised to be one of the more unique events in the life sciences space this coming fall. This full-day investor partnering conference is groundbreaking in that it is focused on redefining the investor landscape in early stage life sciences. As all of us in the industry are aware, the life science investor landscape has changed; venture capital has left a void and there is a plethora of new entities entering the space with capital to allocate.
This conference has assembled these players – Senior decision-makers from some of the largest pharmaceutical & device companies, patient groups, philanthropic organizations, investment banks, and family offices will all be joining the action on September 16th. The conference will also have representation from next-generation technology transfer, licensing and funding experts, and there will be a free fund-raising boot camp. We urge all biotech and medtech readers to take a look at the program, and to take some time out to reeducate themselves regarding the new landscape unfolding in the life science investor arena.
Biohealth Innovation has been able to secure Earlybird pricing for our readership through August 30th via this link – We look forward to meeting you in September!
back to top 
 Kauffman Foundation FastTrac®, has joined with Montgomery College to support future and current business owners before, during, and after the startup process. Entrepreneurs will receive the information, resources, and networks necessary to start and grow successful businesses. Kauffman Foundation FastTrac®, has joined with Montgomery College to support future and current business owners before, during, and after the startup process. Entrepreneurs will receive the information, resources, and networks necessary to start and grow successful businesses.
Three courses will be offered:
- FastTrac NewVenture
- FastTrac GrowthVenture
- FastTrac TechVenture
For more information: Program Flyer
back to top 

University of Maryland (UM) Ventures announced today agreements between University of Maryland, Baltimore (UMB) and five different life sciences companies across the Baltimore/Washington metropolitan region. The companies include Montgomery County-based Rexahn Pharmaceuticals, Baltimore County-based Plasmonix, Prince Georges County-based IGI Technologies, Howard County-based A&G Pharmaceuticals, and Frederick County-based BioAssay Works. These deals are part of UM Ventures’ continual efforts to accelerate technology commercialization, advance industry collaboration, and support projects with commercial value at both the Baltimore and College Park campuses of the university.
“UMB is very excited to collaborate with these companies, each an innovator in its own right,” said Phil Robilotto, Assistant Vice President, Office of Technology Transfer, UMB. “These types of collaborations are at the core of our mission to channel the expertise of our industry partners and highlight our efforts to support the Maryland biotechnology community.”
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
NOT-OD-13-095): Using ASSIST to Prepare and Submit Multi-Project Applications to NIH: Webinar – August 13, 2013
NIH will require electronic submission for all P01, P20, P50 and U19 applications intended for due dates on or after September 25, 2013.
NOT-OD-13-097: Extension of eRA Commons User IDs to Individuals in Graduate and Undergraduate Student Project Roles with Measurable Effort on an NIH Annual Progress Report (PHS2590 & RPPR)
Over the next year the NIH will start requiring an eRA Commons ID for all individuals in graduate and undergraduate student roles who participate in NIH-funded projects for at least one person month or more.
NOT-HL-13-187: Notice of Intent to Publish a Funding Opportunity Announcement for Low-Cost Pragmatic Patient-Centered Randomized Controlled Intervention Trials (UH2/UH3)
The NHLBI, along with other NIH Institutes and Centers, intends to promote a new initiative by publishing a Funding Opportunity Announcement (FOA) to solicit applications to plan and conduct low cost, pragmatic, randomized clinical trials that are integrated into existing clinical practice settings and/or leverage existing electronic patient care resources.
Program Announcement (PA):
PA-13-302: Research Project Grant (Parent R01) (expires September 8, 2016)
The Research Project Grant (R01) supports a discrete, specified, circumscribed project to be performed by the named investigator(s) in areas representing the specific interests and competencies of the investigator(s). The proposed project must be related to the programmatic interests of one or more of the participating NIH Institutes and Centers (ICs) based on descriptions of their programs.
PA-13-292: Behavioral and Social Science Research on Understanding and Reducing Health Disparities (R01) (expires September 8, 2016)
The purpose of this FOA is to encourage behavioral and social science research on the causes and solutions to health and disabilities disparities in the U. S. population.
back to top 

About one year ago GlaxoSmithKline (NYSE: GSK) set up a division to study the electrical impulses along the peripheral nervous system and develop technologies to read, change, or manipulate these impulses to treat acute and chronic diseases. Now it’s launched a $50 million venture capital fund to invest in companies with pioneering technology in this emerging field of bioelectronics, according to a company statement.
The Action Potential Venture Capital fund, which will be based in Cambridge, Massachusetts, takes its name from the electrical signals that pass along the nerves in the body. Problems with the patterns of these impulses are associated with a broad range of diseases.
back to top 

When PharmAthene Inc. merges with Seattle-based Theraclone Sciences Inc., it will become part of a company that has the kind of deal with Big Pharma any drug discovery firm would envy.
Theraclone has a multimillion dollar research and development deal with pharmaceutical giant Pfizer Inc. that could be worth as much as $632 million in license, royalty and other payments to the combined company. Theraclone and PharmAthene are entering into an all-stock merger of equals. The combined company will trade publicly.
back to top 

Leggett Joins O’Malley/Brown Administration in Announcing $630 Million Investment in County’s Transportation Network; Governor Also Announces Additional $400 Million for Construction of Purple Line for Montgomery and Prince George’s
Montgomery County Executive Ike Leggett today joined Governor Martin O’Malley and Lt. Governor Anthony Brown during their announcement that Montgomery County will be receiving $628 million in transportation investments and an additional $400 million for construction of the Purple Line that will benefit both Montgomery and Prince George’s counties. Brown also announced that the Purple Line will be built as a public-private partnership under HB 560, the law he championed to attract private investment for new infrastructure in Maryland.
back to top 

The Johns Hopkins University’s schools of medicine and public health received a $5.8 million federal government grant for research on frailty among older adults. The five-year grant will allow the university to continue research designed to identify the causes of frailty in older adults and speed the development of interventions to slow or stop it.
The grant renews funding of the Johns Hopkins Claude D. Pepper Older Americans Independence Center (OAIC ), a federally designated center of excellence that is one of only 14 such university sites nationwide supported by the National Institute on Aging (NIA ). The centers are named for a longtime Democratic member of Congress who championed support for older adults.
back to top 

The University of Maryland announced Monday that long-time finance professor Alexander J. Triantis has been appointed dean of the Robert H. Smith School of Business.
Triantis, 49, succeeds G. “Anand” Anandalingam, who left the post at the end of June to take a position in London as dean of the Imperial College Business School. Triantis will assume the position Sept. 1.
back to top 

Governor Martin O’Malley called attention to state investments in technology during a visit on July 31 to the University of Maryland BioPark, where he spent time in corporate laboratories with chief executive officers and their employees.
Jay A. Perman, MD, president of the University of Maryland, Baltimore (UMB), joined O’Malley on the tour and at a news conference, where the governor spoke about the importance of supporting the life sciences. O’Malley noted that the state has a plan to invest $1.3 billion in life sciences by 2020 and has increased tax incentives to encourage biotechnology and research and development.
back to top 

The University of Maryland University College announced Wednesday it will be the first in the state’s university system to create a path for students to earn academic credit for learning through “massive open online courses.”
The university is one of the nation’s largest public providers of online higher education with an enrollment of about 93,000 students.
back to top 

Digital health accelerator Healthbox and anchor partner Florida Blue had their priorities straight when they chose the seven companies for their inaugural Florida accelerator program: Address the high populations of seniors and youths in the state.
Companies that do that will have more opportunity to scale and build momentum, they reasoned.
back to top 

No factor defines success and failure for a drug company more than this: Companies that invent more, better drugs at a lower cost do better than those that hemorrhage cash but never get an important product to market. Yet 19 in 20 medicines in experimental development fail, meaning a great many companies fail too.
For years, researchers, including one team from Tufts University and another at Eli Lilly, have estimated the cost of inventing and developing a drug at $1 billion or more. These estimates try to exclude costs not directly related to a drug’s approval and also don’t allow for any comparisons between companies. Last year, for the first time, I did something far cruder: I took the 15-year research spending of a group of big pharmaceutical companies and divided it by the number of new drugs (technically new molecular entities, the Food and Drug Administration’s term for drug molecules that have not been approved in any form for any use previously).
back to top 

The Office of Translational Alliances and Coordination (OTAC) in the Division of Extramural Research Activities (DERA), NHLBI is seeking outstanding candidates for the Business Development Specialist (Health Scientist Administrator) position. The OTAC is charged with accelerating the translation of basic discoveries and innovations into new diagnostics, devices, and therapeutics, and facilitating the development of new technologies via Small Business Innovation Research (SBIR) initiatives. The Office facilitates identification of emerging areas of translational opportunities and provides functional integration by developing interdependent teams that leverage resources and intellect across the NHLBI, and with other NIH Institutes, agencies, and organizations. The OTAC enhances communication and coordination between existing programs, develops and coordinates strategic initiatives and Funding Opportunity Announcements (FOAs), and identifies and capitalizes on synergies to meet and enhance program goals. For more information about the OTAC, please visit http://www.nhlbi.nih.gov/about/dera/otac.
The Business Development Specialist will be responsible for the evaluation of the scientific and technological novelty, business opportunity potential and commercialization merits for research projects in the NHLBI SBIR/STTR portfolio as well as other initiatives in the OTAC. Responsibilities of the position include activities such as portfolio management assistance; drafting solicitations; evaluating the effectiveness of short and long-term SBIR projects, providing advice to the OTAC Director and NHLBI senior-level scientists regarding strategic SBIR technology development; establishing internal and external contacts to foster the development of programs and identification of opportunities for SBIR technology research support and collaboration; facilitating scientific collaborations between NHLBI, NIH, DHHS and other Federal agencies, industry and the private sector; and building public-private partnerships to ensure best practices and exchange information.
back to top 

Kleiner Perkins, the investor behind Google and Amazon, and Accel Partners, best known for its investment in Facebook, are putting $18 million into MyFitnessPal, a little-known digital-health startup that has helped its 40 million-plus users shed a collective 100 million pounds.
Despite those accomplishments, this is the first time MyFitnessPal has raised money from professional investors, which raises the question: Do venture capitalists have any idea what they’re doing in the networked fitness market?
back to top 

You may have a great idea for a new diagnostic, drug, or technology but developing, testing, and commercializing it takes money. One very viable source for a biotech start-ups in the US are SBIR and STTR Small Business Technology Development grants.
These federal grant programs provide essential funds for hundreds of entrepreneurs to get their technology out of the lab into the market. In fact, with awards totaling about $700 million per year, the SBIR/STTR program is one of the leading sources of seed funds for start-ups and small businesses developing new disease and health related technologies.
back to top 

Some hospitals are turning to technology entrepreneurs to reduce readmission rates and avoid penalties imposed under the Affordable Care Act, the Wall Street Journal reports.
Background
Last year, an ACA provision went into effect that allows CMS to penalize hospitals for excess readmissions of Medicare patients.
The penalties are based on the number of heart failure, heart attack or pneumonia patients above the national average who are readmitted within 30 days to an acute-care hospital.
back to top 

For most of us, the blister packs our medicines come in are just temporary barriers to be scratched open with our fingernails or popped open like Chiclets. We usually don’t even pay attention to the tiny, vaguely printed expiration dates tattooed on the silver skin of our aspirin or cough medicine’s packaging; we take for granted that it’s in date.
Yet around the world, billions of people can’t take the expiration dates of their medication for granted. Doing so can, and often is, fatal. A new concept could put an end to that by encapsulating our medicines in strips that change color as they expire, transforming the packaging of dangerously out-of-date medication into a chromatic warning. But will big pharma bring it to market?
back to top 

Money may not make the world go round, but it does keep companies innovating. The Small Business & Technology Development Center has made formal a longstanding relationship with BBCetc., an Ann Arbor-based development consulting company with a specialty in helping businesses secure federal grant funding for commercialization and growth.
The partnership will utilize BBCetc.’s extensive background in federal research grant proposals with the SBTDC’s statewide organization and presence to assist companies with writing proposals specifically for the Small Business Innovation Research and Small Business Technology Transfer federal research grants.
back to top 

Proof of Concept grants are intended to enhance the commercial viability of health-related technologies or concepts developed by non-profit organizations or enhance the competitiveness of early-stage companies for private equity investment. The maximum award is $250,000 in total costs.
Submission and review process:
- Three cycles of pre-proposal review, proposal review, and awards by the LSDF Board of Trustees through August 2014. Up to 32 pre-proposals will be reviewed per cycle.
- Pre-proposals are reviewed every four months and require a presentation and interview.
- Invited proposals are due one month after pre-proposal reviews and reviewed the following month. Proposal reviews require a presentation and interview.
- Awards are made one month after proposal reviews.
- If a pre-proposal results in an invitation to submit a proposal, that invitation is valid only for the proposal deadline immediately following the pre-proposal review.
back to top 

The University of Maryland College Park ranks among the best universities in the nation, but it appears the Terps are known to let their hair down.
College Park also ranks among the top 20 party schools in the country, according to the newly released rankings from the Princeton Review.
back to top 
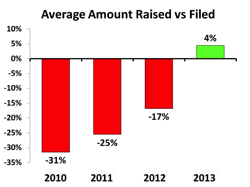
It isn’t just the volume of IPOs this summer that makes this window a victory for hibernating private biotechs and their VC backers, it’s the details of the offerings in terms of price, performance, and type of biotech making the leap onto the public stage. For the last five years we have not seen average offering prices land above expectations, but 2013 is breaking that trend. Price performance is also impressive, with a number of IPOs hovering near 2x the offering price. Rounding out these two positives for valuation is the return of the formerly taboo Phase I and Pre-Clinical IPOs – a qualitative signal of strength.
back to top 

GlaxoSmithKline’s new $50 million venture capital fund will be based out of the One Broadway building in Cambridge’s Kendall Square, inside an office of SR One, the drug giant’s corporate VC arm, a GSK spokesperson said.
The 16-floor One Broadway building also houses the Cambridge Innovation Center startup offices across a number of its floors, as well as venture capital firms Charles River Ventures and Highland Capital Partners.
back to top 

MedCity News writes about all kinds of startups whether their pitch is more at home on CNN or MIT’s Technology Review. We are always so focused on what’s coming next that we forget to reflect on all the companies we have recently discovered. To solve this, we’ve created a monthly report to make sure none of our readers missed any of these posts.
Our new Monthly Startups Index is a free (e-mail registration required) compendium of the early-stage activity across healthcare. It is a piece of business intelligence that includes MedCity’s deeper looks at select early-stage companies, chronicles the investment activity and other news, and even highlights which startups got the most attention from MedCity readers every month.
back to top 

Patients like it and so do health organizations, but electronic communications in clinical care will likely not be widely adopted by primary care physicians unless patient workloads are reduced or they are paid for the time they spend phoning and emailing patients, both during and after office hours.
Those are some key conclusions of an in-depth examination by investigators at Weill Cornell Medical College of six diverse medical practices that routinely use electronic communication for clinical purposes. The detailed report, the most comprehensive of its kind, appears in the August issue of the journal Health Affairs.
back to top 

A person who has suffered from a stroke or spinal-cord injury might need to use crutches or a wheelchair as they gradually regain lost motion through physical therapy.
But these patients could see drastically different effects strapping on a robotic bodysuit or a bionic limb and walking around like Iron Man as they heal. And such digital hardware has the potential to make them recover faster, as well.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 14
William E. Hanna, Jr. Innovation Center

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 12
Novartis Institutes for Biomedical Research

September 18-19
South San Francisco near the Airport
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Emergent BioSolutions Inc. (NYSE: EBS) announced today that it has closed on its acquisition of Bracco Diagnostics Inc.’s Healthcare Protective Products Division. This acquisition, which includes the RSDL® (decontamination lotion) product that is cleared for marketing by the U.S. Food and Drug Administration (FDA) for removal or neutralization of chemical warfare agents from the skin, diversifies and broadens Emergent’s biodefense franchise into the chemical countermeasure market.
“As a result of this transaction closing, Emergent is focused on the uninterrupted supply of RSDL product to customers and on the seamless integration of the new Healthcare Protective Products Group (HPPG) into our biodefense division,” said Adam Havey, EVP and president of Emergent’s biodefense division. “This news, which comes on the heels of other positive developments in our biodefense division, including receiving BioThrax® (Anthrax Vaccine Adsorbed) market authorization in Germany as well as reporting positive data from our pivotal study supporting licensure of a post-exposure prophylaxis indication for BioThrax, reinforces Emergent’s leadership position in the biodefense arena.”
back to top 

Slices from thousands of brains fill nearly a dozen freezers set to 112 degrees below zero on the top floor of a research building at the Johns Hopkins Science + Technology Park.
Inside those slices might be the secrets to schizophrenia and other mental illnesses. On a recent morning, researchers with the Lieber Institute for Brain Development performed dissections of the amygdala, an almond-shaped mass of neurons thought to play a key role in such diseases.
back to top 

Kolltan Pharmaceuticals, a privately held biopharmaceutical company, today announced an agreement with MedImmune, the global biologics research and development arm of AstraZeneca, under which Kolltan will in-license a monoclonal antibody targeting the Her3 receptor tyrosine kinase from MedImmune. Based on the current program status, Kolltan anticipates initiating Phase 1 clinical testing of this product in the first quarter of 2014.
“This in-licensing opportunity exemplifies our leadership in and focus on receptor tyrosine kinases and their key role in oncology and other serious diseases,” commented Dr. Jerry McMahon, Kolltan’s President and Chief Executive Officer. “Kolltan will apply its extensive scientific expertise and R&D drug development experience to prepare for entry into the clinic, including a focus on patient selection strategies. Kolltan is excited to advance this innovative product candidate into clinical testing for the broad potential treatment of cancer patients where this target plays a role.”
back to top 

Maryland’s venture capital fund has announced that two tech companies in the state will get a total of $350,000.
Gov. Martin O’Malley made the announcement on Monday.
A Baltimore firm called SocialToaster has received approval for $200,000. The company specializes in social media and customer engagement.
back to top 

BD Diagnostics, a segment of BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, and the College of American Pathologists (CAP), the world leader in laboratory quality assurance and improvement, today announced the launch of a new strategic alliance that will provide solutions to advance laboratory quality for improved patient outcomes in China and India. BD and CAP announced the collaboration during the American Association for Clinical Chemistry (AACC) Annual Meeting in Houston, Texas.
Laboratories play a critical role in the diagnosis and treatment of disease for the more than 2.5 billion people who live in China and India. The BD/CAP Strategic Alliance will improve access to external quality assurance/proficiency testing (PT) that can have a direct and positive impact on laboratory quality, and therefore, patient outcomes. Together BD and CAP will provide education to improve awareness of global practice standards and training that will help laboratories achieve their quality improvement goals. Additionally, BD will manage PT distribution, including sales, shipping, and first-line client service.
back to top 

Becton, Dickinson and Company is one of domain players of insulin syringes and other delivery devices, referring to the pie chart, Market Shares of the leading suppliers of Insulin Syringes and other delivery devices, 2009. Today, the market shares accounts 45% in the world, 80% in U.S. and Europe .
back to top 
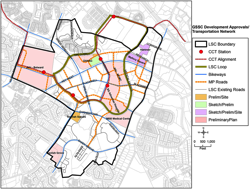
Research organizations in the Great Seneca Life Sciences Corridor are considering the creation of a common cyberinfrastructure that would facilitate sharing and spur innovative ideas.
Anil Srivastava, president of Open Health Systems Laboratory on Johns Hopkins University’s Montgomery County campus, proposed the idea and held a meeting Monday with physicians, university faculty and representatives from Cisco Systems, Montgomery County government, the National Institute of Standards and Technology, MedImmune and other organizations.
back to top 

The University of Maryland University College announced Wednesday it will be the first in the state’s university system to create a path for students to earn academic credit for learning through “massive open online courses.”
The university is one of the nation’s largest public providers of online higher education with an enrollment of about 93,000 students.
back to top 

The founders of District startup hub 1776 have shopped around the idea of raising a $25 million seed fund — modeled partially off of 500 Startups — that would place initial bets as high as $150,000 on early-stage tech companies, according to an investor presentation obtained by the Washington Business Journal.
1776 co-founder Evan Burfield cautioned that the document is a draft, and that 1776 is not actively raising capital yet for the planned seed fund and accelerator. He stressed that officials there haven’t arrived on a target for the size of the fund and that $25 million — and other figures in the document — are just one of several ideas they’ve contemplated.
back to top 

There’s a “No Vacancy” sign hanging out front University of Maryland, Baltimore County’s research and development park, and leaders say they have the state’s cyber security industry to thank.
University officials say the popularity of cyber security and growth in the number of young companies launching in that industry contributed to bwtech@UMBC’s popularity in recent years. The research and technology park is at capacity for the first time since it was established in 1989.
back to top 
Register now for the 3rd Annual Maryland Cyber Challenge
Live Finals! October 8 and 9, 2013 at the Baltimore Convention Center
- Three levels of team competition:
- high school (network defense)
- college, and professionals (capture the flag)
- Open to competitors across the United States, travel costs for finals are the team’s responsibility
- Lock in early for $50 discount team member names must be finalized by Monday, Sept. 9
- Competition schedule includes practice rounds, qualification rounds and a final cram session
- Team registration includes conference pass per team member and coach
back to top 

“Deidentified” data on individual participants in more than 200 GlaxoSmithKline-sponsored clinical trials are now available to independent researchers, company officials said.
And by the end of the year, the database is likely to include patient-level data from some 400 interventional trials, according to Perry Nisen, MD, PhD, and Frank Rockhold, PhD, of GSK’s research division in King of Prussia, Pa.
back to top 
Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Requests for Applications (RFA):
back to top 

The National Institutes of Health will fund up to $24 million per year for four years to establish six to eight investigator-initiated Big Data to Knowledge Centers of Excellence. The centers will improve the ability of the research community to use increasingly large and complex datasets through the development and distribution of innovative approaches, methods, software, and tools for data sharing, integration, analysis and management. The centers will also provide training for students and researchers to use and develop data science methods.
Biomedical research is increasingly data-intensive, with researchers routinely generating and using large, diverse datasets. Yet the ability to manage, integrate and analyze such data, and to locate and use data generated by others, is often limited due to a lack of tools, accessibility, and training. In response, NIH launched the Big Data to Knowledge (BD2K) initiative in December. This initiative supports research, implementation, and training in data science that will enable biomedical scientists to capitalize on the transformative opportunities that large datasets provide. The investigator-initiated BD2K Center of Excellence funding opportunity is the first of several BD2K funding opportunities to be announced in coming months.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
NOT-HL-13-183: Notice of Information: NHLBI Will Only Accept Resubmitted Applications for the Extended Expiration Date for PAR-10-234 Bioengineering Research Partnerships (BRP)(R01)
- The NHLBI will only accept resubmitted applications for the November 5, 2013 and January 7, 2014 (AIDS) standard due dates. Furthermore, all resubmission applications must come in on November 5, 2013 or January 7, 2014 (AIDS). NHLBI will not accept new, renewal or revision applications on November 5, 2013 and January 7, 2014.
NOT-OD-13-083: Extramural Loan Repayment Program for Pediatric Research (LRP-PR)
- The NIH invites qualified health professionals who contractually agree to engage in NIH mission-relevant research for at least two years, and who agree to engage in such research for at least 20 hours per week based on a 40-hour work week, to apply for participation in the extramural LRP.
NOT-OD-13-081: Extramural Loan Repayment Program for Clinical Researchers (LRP-CR)
- The NIH invites qualified health professionals who contractually agree to engage in NIH mission-relevant research for at least two years, and who agree to engage in such research for at least 20 hours per week based on a 40-hour work week, to apply for participation in the extramural LRP.
Request for Applications (RFA):
RFA-HL-14-001: Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases Stage II (CADET II)(UH2/UH3)
- The purpose of this Funding Opportunity Announcement (FOA) is to invite applications for Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases Stage II (CADET II). The goal of the CADET program is to accelerate the development of novel products for the treatment of lung diseases and sleep disordered breathing using strategies based on relevant pathobiologic processes.
back to top 

“All of us in the life sciences, medical device, and consumer industries are trying to get better at collaboration,” observed Robert Urban, PhD, head of the Johnson & Johnson (J&J) Innovation Center in Boston. For the Boston Innovation Center, which opened June 27, “getting better” includes enormous flexibility in the details of the early stage collaborative deals it inks with individual entrepreneurs, small companies and research institutions.
“A key focus is real adaptability. We strive to build collaborations that are unique to particular opportunities, based upon their specific needs. We have no preconceived notions regarding collaboration. Everything’s on the table,” Urban emphasized. Consequently, there’s no one typical deal or collaboration model. Instead, J&J’s team of business, scientific and transaction experts looks at each individual opportunity during their early stages to determine feasibility, fit within J&J’s goals and the best way to move forward.
back to top 

Rhode Island’s capital city should forget about becoming another Silicon Valley, venture capitalist Josh Kopelman said during a talk Tuesday at Betaspring, the business incubator in the Knowledge District.
It’s an unrealistic goal for Providence and the many other cities that aspire to become the nation’s next technology hub. But, said Kopelman, the founder and managing partner of First Round Capital, that doesn’t mean those communities can’t carve out their own niche in an industry that’s growing at a feverish pace.
back to top 

When Qiagen scooped up Ingenuity Systems this year, the acquisition of the Redwood City, California-based firm marked the first time the biotechnology giant had purchased a firm that exclusively makes software.
The purchase allows Qiagen to analyze information it derives from the genetic maps of organisms, which can be used to detect variations and mutations that point to the cause of certain diseases or new ways to treat them.
back to top 

Innovation is a hot topic in organizations today. And no wonder. Customers want new and different. Costs can’t be reduced any further. Business pressures abound. The economic picture is spotty. Competition is fierce.
Innovation to the rescue!
back to top 

A new class of medicines could give doctors the ability to awaken underperforming genes in patients who currently have no treatment options.
Boston-area startup RaNA Therapeutics is developing a novel kind of medicine that can boost the activity of genes that may be silenced or underactive and thus cause disease. The medicine would use a small RNA-like molecule that blocks the function of a long RNA molecule that is hampering the expression of such a gene.
back to top 
 The RESI conference is poised to be one of the more unique events in the life sciences space this coming fall. This full-day investor partnering conference is groundbreaking in that it is focused on redefining the investor landscape in early stage life sciences. As all of us in the industry are aware, the life science investor landscape has changed; venture capital has left a void and there is a plethora of new entities entering the space with capital to allocate. The RESI conference is poised to be one of the more unique events in the life sciences space this coming fall. This full-day investor partnering conference is groundbreaking in that it is focused on redefining the investor landscape in early stage life sciences. As all of us in the industry are aware, the life science investor landscape has changed; venture capital has left a void and there is a plethora of new entities entering the space with capital to allocate.
This conference has assembled these players – Senior decision-makers from some of the largest pharmaceutical & device companies, patient groups, philanthropic organizations, investment banks, and family offices will all be joining the action on September 16th. The conference will also have representation from next-generation technology transfer, licensing and funding experts, and there will be a free fund-raising boot camp. We urge all biotech and medtech readers to take a look at the program, and to take some time out to reeducate themselves regarding the new landscape unfolding in the life science investor arena.
Biohealth Innovation has been able to secure Earlybird pricing for our readership through August 30th via this link – We look forward to meeting you in September!
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 14
William E. Hanna, Jr. Innovation Center

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 12
Novartis Institutes for Biomedical Research

September 18-19
South San Francisco near the Airport
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
BHI Also Announces Agreement With BD to Create Entrepreneur-in-Residence Position

Rick Ivey, BD Diagnostics
BioHealth Innovation, Inc. (BHI) today announced the fiscal year 2013-2014 election of officers and a new appointment to its Board of Directors. BHI also announced it has entered into an agreement with BD (Becton, Dickinson and Company) to establish an entrepreneur-in-residence (EIR) position at the National Institutes of Health (NIH) Office of Technology Transfer. In conjunction with this agreement, BD is entitled to a voting position on the BHI Board of Directors, which will be held by Richard M. “Rick” Ivey, Worldwide Vice President Research & Development, BD Diagnostics – Diagnostic Systems.
“I am pleased to welcome the new roster of officers and a new member to the BHI Board of Directors,” said Richard Bendis, BHI President and CEO. “The officers are a committed group of individuals who already have contributed to the steady growth of BHI, and will continue to be important leaders as the organization further develops.”
“Rick Ivey joins the Board on behalf of BD as part of the terms of an agreement between BHI and BD to establish an NIH EIR position,” added Mr. Bendis. “He represents an important new addition to our Board as he is a seasoned medical technology executive who can offer experience and insights to the growing cadre of start-up diagnostics companies in the State of Maryland.”
back to top 

The state of Maryland normally goes unnoticed in regards to the medtech and bioscience sectors when compared to California, Massachusetts and Minnesota. However, the state has the research facilities, governmental institutions and programs, corporate partnerships, funding and ambition to advance its biosciences marketplace into an international hub for medtech innovation.
At the heart of Maryland’s medical device and broader biotech industries is the state’s network of institutes and universities, which includes the National Institutes of Health, the National Institute of Standards & Technology, Johns Hopkins University and the University of Maryland. Each one of these entities boasts an array of specialized laboratories for the study of bioimaging, biomolecular modeling, drug delivery, biomaterials, MEMS, microfluidics and more.
back to top 

Montgomery County Executive Ike Leggett is leading a 2013 trade and Sister-City China Mission trip from Sept. 15 to Sept. 25, 2013. The Mission is organized by Montgomery County Government and Maryland China Business Council ( MCBC ) with the support of the State of Maryland ’s Maryland Center China ( MCC ) in Shanghai, which has been assisting local companies in China since 1996. This combination of experience and long term relationships offers a unique opportunity to explore, enter or expand in one of the world’s most dynamic and fast growing markets and destinations. Mission Statement:
Sister Cities create relationships based on cultural, educational and trade exchanges, creating lifelong friendships that provide prosperity and mutual benefits through “citizen diplomacy.”
Participants for trip include:
County Executive; State and County Government Representatives; Business, Education, Academic and Science Leaders; Chinese Community Leaders
back to top 

Topic: “EIRs, SBIRs, and more with BioHealth Innovation, Inc.”
Presenters:
- Richard Bendis, President and CEO
- Ethan Byler, Director, Innovation Programs
- Todd Chappell, Entrepreneur-in-Residence, NIH-OTT
- Dr. Ken Malone, Entrepreneur-in-Residence, UMD Ventures
- Ram Aiyar, Entrepreneur-in-Residence, NHLBI
BioHealth Innovation, Inc. (BHI) is a regional innovation intermediary that accelerates and facilitates technology transfer and commercialization of market-relevant research in federal labs, universities, and biohealth companies in the Region. It is a private-public partnership that connects the Region’s innovation assets to provide integrated technical knowledge, financial means, and entrepreneurial/managerial expertise to turn promise into prosperity for the region while advancing human health.
BHI’s Entrepreneur-in-Residence (EIR) program is designed to be an active partner with research institutions to source, fund, and grow high-potential, early-stage products through project-focused companies. The entrepreneurs in the program support the formation of new companies based upon innovative discoveries in the areas of drugs, vaccines, therapeutics, diagnostics, and medical devices from the intramural research programs at the NIH and Food and Drug Administration (FDA), as well as from universities and businesses.
BHI’s Commercial Relevance Program (CRP) offers biohealth companies support in preparing applications for federal funding inclusive of SBIRs, STTRs, and other federal government awards. Companies submit their federal funding concepts and receive pre-proposal feedback to help troubleshoot and strengthen your application. Further support from professional consultants and service providers is available to assist in improving your application.
BHI recently published the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide serves as a compendium of resources to biohealth innovators and entrepreneurs working to start and grow new companies and technologies in the region.
back to top 

The bridges had to be strong and stable — and made of spaghetti.
Charged with this unusual engineering task, a group of about 40 high school students worked intently Thursday with the uncooked yellow strands to build a roughly half-meter structure they had designed to support more weight than their competitors’.
back to top 

QIAGEN N.V. announced a partnership with Exosome Diagnostics Inc. to develop and commercialize high-performance sample preparation kits for the processing of nucleic acids from exosomes.
According to a release, combining the Exosome Diagnostics platform technology approach with select QIAGEN consumables and automation platforms has the potential to allow researchers, drug developers and doctors to take repeated, real-time genetic “snapshots” of disease from patients’ blood, urine or cerebrospinal fluid without the need for tissue biopsy. The companies are targeting initial product launches in the first half of 2014. Financial terms were not disclosed.
back to top 

The Daily Record is proud to announce its 2013 Most Admired CEO award winners.
The award recognizes men and women who have excelled professionally and in serving their communities.
The nominees were evaluated in terms of leadership and vision, competitiveness and service, community leadership and service, financial performance and growth and corporate leadership and board service.
back to top 

In 2012, Maryland ranked 5th in the number and total value of Small Business Innovation Research/Small Business Technology Transfer (SBIR/STTR) program awards, after California, Massachusetts, Virginia and New York. Maryland firms received 265 awards valued at $94 million. A DBED analysis of SBIR/STTR Phase I and Phase II data shows that the number and value of awards decreased nationally in the last four years, and Maryland followed this trend. Even so, when compared to other states, Maryland consistently ranked within the top five recipients of SBIR/STTR awards in both the number and the dollar value of awards received.
back to top 

Montgomery County’s Department of Economic Development is hoping to attract green businesses to the area with an incentive program that will reimburse investors as much as $25,000.
The Green Investor Incentive Program is now accepting applications from investors in businesses that provide green products or technologies. The program was approved in April by the County Council and County Executive Isiah Leggett appropriated $500,000 to fund the program.
back to top 

Regular marijuana use in adolescence, but not adulthood, may permanently impair brain function and cognition, and may increase the risk of developing serious psychiatric disorders such as schizophrenia, according to a recent preclinical study from the University of Maryland School of Medicine.
Researchers hope that the study, published in Neuropsychopharmacology — a publication of the journal Nature – will help to shed light on the potential long-term effects of marijuana use, particularly as lawmakers in Maryland and elsewhere contemplate legalizing the drug.
back to top 

Karen Vignare, Ph.D., an innovator in curriculum design and in the use of technology in learning, has been named associate provost and will lead University of Maryland University College’s new Center for Innovation in Learning. Vignare began her new post on June 3.
The Center for Innovation in Learning will be a laboratory for continuous improvements to the university’s curriculum, faculty development model and student support through its own work and through partnerships with high-profile organizations that will help further the work of the center.
back to top 

Washington, DC, is growing as a close-knit entrepreneur community where startups can find angel investors who will also act as mentors to grow a business, venture capitalists say.
The DC area has fewer venture capital firms than Silicon Valley, but steady startup investment is coming from tech industry veterans who have experience working with area universities, government agencies, and telecom companies including AOL, said John Taylor, head of research for the National Venture Capital Association (NVCA).
back to top 

What Is Baltimore Innovation Week?
Baltimore Innovation Week is a week-long celebration of technology and innovation in Baltimore. The annual week of events is intended to grow the impact of this innovative region through programming focused on technology, collaboration and improving Baltimore.
Last year, Baltimore Innovation Week 2012 had impact:
- More than 30 events from 25 partners with more than 1,500 attendees
- Startups and products launched, including NewsUp, Crowdstich and Easy WebContent’s Presenter.
- GBTC held Maryland’s first hackathon that featured representatives from city, state and federal government agencies.
- 15 projects launched as part of the latest Startup Weekend Baltimore, including winner TeamPassword.
- Venture for America unveiled plans to launch its fellowship program in Baltimore.
- TechBreakfast continued its growth as the region’s premiere startup demo event.
- Pitch Across Maryland brought Gov. O’Malley to Baltimore to show off his entrepreneurship-fueling work.
- The University of Maryland Biopark launched a new entrepreneurship demo series, starting with its incubated healthcare applications platform startup Analytics Informatics.
- Education Ignite welcomed more than 200 people to hear about the changing education climate in Baltimore.
back to top 

In the downtown of the nation’s capital, there is a magnificent building of steel and glass that is now home to what may be a remarkable tech experiment.
The D.C. Public Library took an 11,000-square-foot space and installed 80 computers, including 16 Macs. A 3D printer was added as well as a machine that can print and bind a book from a file in just minutes. There are tablets of all types — Android, Windows, Apple — and e-reading devices, available to try out. It opened last week.
back to top 

Independence Blue Cross (IBC), Penn Medicine, and DreamIt Ventures today host “Demo Day” for 10 health care startup companies selected to participate in DreamIt Health, the first-ever Philadelphia-based health care accelerator. The event will be held from 10 a.m. to 2 p.m. at World Café Live! at 3025 Walnut Street in Philadelphia. The participants will each have seven minutes to present their business plans to potential investors and customers, mentors, and health care executives. Demo Day events are ideal opportunities for startups to begin raising money to launch their businesses and gauge interest among key audiences.
“As health care undergoes dramatic change, I believe that the health care companies that thrive will be those with a clear vision that move rapidly and innovate. The caliber of this group of entrepreneurs and the diverse health care companies they represent is very promising and we’re pleased to have played a part in helping them plan, develop, and now present their ideas,” said Daniel J. Hilferty, president and CEO of IBC. “We are committed to transforming our region into a magnet for health care innovation, investment and employment, and supporting bright, new ideas like those we nurtured through DreamItHealth will help us get there faster.”
back to top 

Accelerators are all about discovering and mentoring talented entrepreneurs with good ideas for improving healthcare and other sectors. They make the right introductions to advisers, industry stakeholders and investors with the goal of improving the chances of their success. But when demo day ends, the team members are faced with the crucial question of how to sustain themselves as they advance the development of their companies. They need to figure out where their next sources of funding will come from.
back to top 

No, you didn’t fall off the list, your SBIR Insider has been silent since February 28, 2013. There is a lot of SBIR news to report but first I owe you an explanation of my absence.
Those of you who are “mature” enough to remember the ever garrulous Howard Cosell, may recall the Larry Holmes / Tex Cobb fight in 1982 that ended Cosell’s illustrious boxing commentary career. Because the fight was so one sided, potentially tragic, a public mutilation, and just plain ugly (with the referee refusing to stop the fight), Cosell became so incensed that he fell mute from the middle of the fight on, never to announce another bout.
back to top 

Three more biotechnology companies went public Thursday as startups continued to capitalize on strong demand for these offerings, a trend that’s giving life-sciences venture capitalists a much-needed lift.
Cellular Dynamics International Inc., Conatus Pharmaceuticals Inc. and Onconova Therapeutics Inc. debuted on Nasdaq Thursday. Their initial public offerings followed recent IPOs from venture-backed biotechs such as Agios Pharmaceuticals Inc., bluebird bio Inc., Esperion Therapeutics Inc., OncoMed Pharmaceuticals Inc. and Prosensa Holding BV.
back to top 

A new mobile health trends report released Wednesday underscores the mid- to low sophistication of current mHealth application technology but also emphasizes the explosive growth and integration headed for the market .
The Research and Markets mHealth trends report shows the industry poised for a compound annual growth rate of 61 percent by 2017, to reach a value of $26 billion. This revenue, researchers project, will be derived predominantly from mHealth hardware sales and services.
back to top 

Medtronic has entered into an innovative partnership with The Johns Hopkins University, agreeing to provide $200,000 a year for up to three years and skilled mentoring to help biomedical engineering students design new healthcare solutions for underserved patients in developing countries.
The partnership was announced recently by Omar Ishrak, Medtronic’s chairman and chief executive officer, during his keynote address at the university’s annual Biomedical Engineering Design Day event. During his talk, Ishrak called attention to the need to improve access, outcomes, and the efficiency of healthcare solutions in developing regions of countries such as India, China, and Brazil.
back to top 

“Proper email is a balance between politeness and succinctness,” entrepreneur-investor-author Guy Kawasaki tells Entrepreneur.com. “Less than five sentences is often abrupt and rude, more than five sentences wastes time.”
In this way, the email is like poem. A sonnet maybe, with the way its limitations have a funny way of granting freedom. Or maybe an epic poem, given the fact that we all write a novel’s worth of email every year. But would a missive by any other length read just as sweet?
back to top 

Given poorly, criticism tends to lead to the criticized parties involved feeling like crap–and the criticizer looking like a jerk (or worse).
If you’re a psychologist, you’d call it reactance. If you’re a regular person, you’d call it a dick move.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 14
William E. Hanna, Jr. Innovation Center

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 12
Novartis Institutes for Biomedical Research

September 18-19
South San Francisco near the Airport
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

A Silver Spring company is looking to build the county’s first net zero facility, a building that would produce enough energy to sustain itself annually.
United Therapeutics —a biotechnology company that works on the development and commercialization of unique medical products — is expanding its campus with the new facility on the corner of Spring Street and Colesville Road in downtown Silver Spring. The building at 1000 Spring Street will have solar panels, a green roof and special placement of windows to allow for natural light and cross breezes to push hot air out of the facility, among other environmentally sustainable features, according to a presentation the design team gave to the Silver Spring Citizens Advisory Board on July 8.
back to top 

Rexahn Pharmaceuticals, Inc. (NYSE MKT: RNN), a clinical stage biopharmaceutical company, announced today that it has signed an exclusive license agreement with the University of Maryland, Baltimore (UMB) for a novel drug delivery platform, Nano-Polymer-Drug Conjugate Systems (NPDCS). This technology targets the delivery of currently marketed chemotherapeutic agents directly into cancerous tumors. The direct delivery of chemotherapeutic drugs into the tumors has been shown to result in increased efficacy and reduced toxicity.
The NPDCS platform combines existing chemotherapeutic agents with a proprietary polymer carrier that contains a signaling moiety which directs the drug into the tumor. This approach minimizes the levels of freely circulating anti-cancer agents in the body, which can dramatically reduce potential adverse events, and maximizes anti-tumor activity by accumulating in the cancer tumor. NPDCS is a broad platform that has the potential to generate multiple therapeutic candidates going forward.
back to top 

Emergent BioSolutions Inc. (NYSE:EBS) today announced the appointment of General George A. Joulwan (retired) to the company’s Board of Directors. General Joulwan has a highly distinguished military career that spans 36 years from 1961 to his retirement in 1997. Highlights of General Joulwan’s military service include: Serving as Supreme Allied Commander Europe (SACEUR); Commander in Chief, U.S. Southern Command; Commanding General, V Corps and Commanding General, 3rd Armored Division, United States Army Europe and U.S. Seventh Army, Germany. He has received numerous military decorations and foreign awards and decorations for his bravery and service, including two Silver Stars for valor.
Fuad El-Hibri, executive chairman of the board of Emergent BioSolutions, stated, “General Joulwan has devoted his four-decade career to serving the country, protecting our freedoms, and architecting peace around the world. He is a true and distinguished public servant, who is highly-respected in the global military community. As we expand our portfolio with specialized products that address the needs of U.S. and worldwide governments, his expertise and stature will be invaluable in guiding Emergent’s management team towards further growth.”
back to top 

Overseas medical technology companies continue to stream into Greater Boston, lured by the area’s famous ecosystem of researchers, startups, and potential collaborators.
One company that flew in under the radar was Qiagen N.V., a Dutch holding company with corporate offices in Germany, which quietly acquired two privately held Massachusetts companies last year and may—or may not—be expanding its foothold in the Boston area.
back to top 

Montgomery Business Development Corporation is pleased to announce the launch of their new website montgomerybusiness.org.
The enhanced website includes business-friendly features, data resources and information to support existing and future business and development in our vibrant economy.
back to top 

About 20 kids garbed in lab coats, booties and goggles entered a laboratory on Friday through a door marked with a bright-red “BIOHAZARD” sticker.
Filling the small room, they gathered around lab coordinator and microbiologist Cindy Reichelderfer, who held up several petri dishes in which scientists had tested for the presence of anthrax.
back to top 
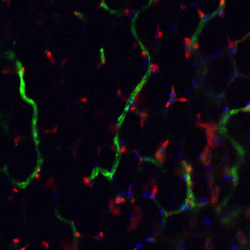
Johns Hopkins researchers have coaxed stem cells into forming networks of new blood vessels in the laboratory, then successfully transplanted them into mice, a technique that could potentially be used to make blood vessels genetically matched to individual patients, the investigators say.
Their research results appear online this week in the Proceedings of the National Academy of Sciences.
back to top 

The Maryland Industrial Partnerships (MIPS) program, an initiative of the Maryland Technology Enterprise Institute (Mtech) in the A. James Clark School of Engineering at the University of Maryland, has awarded $3.8 million to 17 teams combining Maryland companies with university researchers to bring technology products closer to market, program officials announce today.
MIPS, a technology acceleration program, grants money matched with company funds to faculty engaged in each project.
back to top 

The Maryland Technology Development Corp. (Tedco) has invested $1.1 million in 14 state startups.
The grants were made through the organization’s Technology Commercialization Fund. The money will be put toward advancing the each company’s technology and product commercialization efforts.
back to top 

Johns Hopkins Hospital has reclaimed the top position on U.S. News & World Report’s 2013-2014 list of best hospitals.
The return to No. 1 comes a year after Massachusetts General Hospital edged out Hopkins for the top spot on last year’s honor roll list. Hopkins had been No. 1 since 1991.
back to top 

Venture capitalists invested $318 million in young Maryland companies from April through June, an increase of 115 percent from the first quarter, according to a report released Friday by PricewaterhouseCoopers.
About half of that financing, or $150 million, went to Precision for Medicine Inc., a Chevy Chase company that provides services for medical drug discovery, the report said. That was the highest amount any business in the country raised in the quarter and was matched only by a New York e-commerce website.
back to top 

The D.C. region raised $418 million in venture funding in April, May and June, according PricewaterhouseCoopers’ MoneyTree report, more than twice the total of second quarter 2012.
The “DC/Metroplex,” as defined by PWC, includes far-flung areas of Virginia and Maryland, not just the District and its suburbs. With the strong second quarter haul, the region has raised nearly as much in the first half of 2013 ($704 million) as it did all of last year ($735 million). For comparison, companies in the area raised $203 million in Q2 2012, and $286 million in the first three months of 2013.
back to top 

NCATS Research & Development Day will provide the unique opportunity to showcase the projects and technologies that have been incubating in a variety of NCATS drug development programs, including Therapeutics for Rare and Neglected Diseases (TRND) and Bridging Interventional Development Gaps (BrIDGs), to an audience of biopharmaceutical companies, venture capital, angel investors, foundations, and others. The object is to connect our collaborators with strategic partners that will provide financial and technical support to bring potential novel therapeutics to patients. The event will be held:
Thursday, September 12, 2013
Novartis Institutes for Biomedical Research
Cambridge, Massachusetts
back to top 

When: Wednesday July 24, 2013 from 4:30 PM to 7:00 PM EDT
Where: Growlers 227 E Diamond Ave Gaithersburg, MD 20877
Join us for a co-hosted BioBuzz and Women In Bio event with our sponsor, Social & Scientific Systems, Inc., along with many others from the local biotech industry at another exciting BioBuzz event on July 24th from 4:30 – 7:00 p.m. in Gaithersburg. Due to an overwhelming positive response to the location, we’re continuing to holding the event this month at Growlers in Old Towne Gaithersburg. We’re excited to see all of you soon, so please register today!
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
NOT-OD-13-087: Notice of Change to Page Limits and Application Due Date in RFA-OD-13-008 Limited Competition: Restoring Research Resources Lost Due to Hurricane Sandy (R24)
- The purpose of this Notice is to revise the page limits for the Research Strategy section and to extend the due date of RFA-OD-13-008.
NOT-DK-13-012: Notice to Include AIDS Application Due Dates for PA-12-179 Exploratory/Developmental Clinical Research Grants in Obesity (R21)
NOT-HL-13-184: Correction of Key Dates for PAR-13-009 Secondary Dataset Analyses in Heart, Lung, and Blood Diseases and Sleep Disorders (R21)
- Key dates for PAR-13-009 “Secondary Dataset Analyses in Heart, Lung, and Blood Diseases and Sleep Disorders (R21) have been revised.
Requests for Applications (RFAs):
RFA-HL-14-010: Developing a Point-of-Care Device for the Diagnosis of Sickle Cell Disease in Low Resource Settings SBIR (R43/ R44)
- This announcement encourages applications that propose to develop a point of care device for the diagnosis of sickle cell disease (SCD) in infants and young children in low-income and low-resource settings. The aim of this program is to provide rapid diagnosis of SCD to children such that appropriate therapy can be given to reduce the risk of future complications.
back to top 

Monday, August 19, 2013 – Wednesday, August 21, 2013
Time: 7:00 a.m. – 5:00 p.m.
Location:
Natcher Conference Center
45 Center Drive
Bethesda, MD 20892
Sponsored by:
Blood Diseases Program
Division of Blood Diseases and Resources
National Heart, Lung, and Blood Institute
National Institutes of Health
Department of Health and Human Services
back to top 

National Cancer Institute scientists have released the largest-ever database of cancer-related genetic variations, providing researchers the most comprehensive way so far to figure out how to target treatments for the disease.
Open access worldwide to the new database, based on genome studies, is expected to help researchers accelerate development of new drugs and better match patients with therapies, NCI said in a statement on Monday.
back to top 

We often hear public leaders say “our diversity is our strength,” which has become somewhat a cliché over the years. While I don’t doubt their sincerity in believing what they say, I wonder how many truly understand what it means to have a large, diverse, and global population in their communities.
This past weekend, I attended the Chinese Biopharmaceutical Association’s (CBA) 18th annual conference, which attracted scientists, educators, businesses and entrepreneurs from the region as well as delegations from several cities in China. It was a high-energy conference hosted by an all-volunteer crew of local community members.
back to top 

If there’s one thing everyone in healthcare can probably agree on right now, it’s that there is an awful lot of data being generated each and every day. What to do with that data, however, is another question.
As Ted Driscoll, digital health director at venture capital firm Claremont Creek Ventures, sees it, the explosion of data is a definite boon for personalized medicine. Indeed, he said recently, “Medicine is becoming synonymous with big data – the data sets are just huge, now – but we had to wait for the IT revolution to happen and mature” in order to begin to put that data to use.
back to top 

The year is only half over, but one of the biggest biotech stories of 2013 is going to be the resurgence of the biotech IPO market. It’s a good news/bad news story, depending on where you stand, and how far you look out into the future.
First, the good. The IPO surge is a vote of confidence in biotech from generalist investors who have spent years ignoring the industry. It’s good news for biotech entrepreneurs and venture capitalists who back them. A lot of money will get pumped into researching and developing drugs for diseases that have been long neglected, like Duchenne Muscular Dystrophy. Regional innovation clusters will get a boost. Many small companies will have more negotiating leverage when they talk to Big Pharma companies about acquisitions. It might spur more much-needed venture investment in biotech startups.
back to top 

Biotechnology companies can access financial and management resources through venture capitalist (VC) firms. An analysis of 1,490 VC investments shows that country-of-origin (CO) of biotech companies has an effect on the participation by VC firms in various biotech subsectors. Specifically, it is found that US biotech companies tend to have higher amount received per VC firm, greater number of VC firms investing in them and greater biotech investment experience of the investing VC firms. Asia-Pacific biotech companies have consistently less VC firms investing in them and these investing VC firms tend to have less biotech investment experience. VC firms with greater biotech investment experience are also investing in European biotech companies more than those from the Americas less US. CO also correlates with outcomes in the four of the six key biotech subsectors studied. These findings suggest a strong CO effect of VC investment in biotech companies.
back to top 

“We are a team of guerrilla fundraisers who have launched a global campaign to fund research into a potential treatment for the cancer that killed Steve Jobs. The potential therapy, a cancer-busting virus, is currently sitting in a freezer in Sweden – but it can’t be tested for lack of just £2million” was iCancer’s pitch on Indiegogo, a crowdfunding portal. The company brought in more than $160,000 from this campaign.
Microryza is another crowdfunding platform exclusively for scientific research projects, available only to PhDs and professors who can attempt to raise money through this private channel instead of applying for grants. “This solution helps close the gap for potential and promising, but unfunded projects,” Bill Gates says about Microryza. With Kickstartr’s popularity, there has been an explosion of growth in crowdfunding portals, both general as well as ones targeting a specific niche.
back to top 

When it comes to lending to tech startups, Silicon Valley Bank has an impressive grip on the market. The Santa Clara, Calif.-based institution has been in the D.C. area 15 years and claims half of the region’s venture-backed companies as its clients. The bank, which has $21.5 billion in assets, has grown its Tysons Corner team by 25 percent in the past year to 10 people. I caught up with Megan Scheffel, who manages the bank’s Mid-Atlantic and Southeastern U.S. regions.
back to top 

Here’s another reason the dysfunctional federal budget process is bad for Americans: besides hurting the economy and hitting us in the pocketbook, partisan feuding over budget cuts could undermine our health and even shorten our lives.
That’s because House Majority Leader Eric Cantor and others in Congress have been using the budget process to target research in the behavioral and social sciences for elimination, even though they’re indispensable to understanding and improving Americans’ health.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

A total of 150 investors are vying for Maryland’s biotechnology tax credit this year, compared with 125 applicants last year.
Maryland’s Biotechnology Investment Incentive Tax Credit program gives tax credits to individuals and companies that invest in Maryland-based biotechnology startups. The program will give out $10 million in tax credits for fiscal 2014.
back to top 
 Kauffman Foundation FastTrac®, has joined with Montgomery College to support future and current business owners before, during, and after the startup process. Entrepreneurs will receive the information, resources, and networks necessary to start and grow successful businesses. Kauffman Foundation FastTrac®, has joined with Montgomery College to support future and current business owners before, during, and after the startup process. Entrepreneurs will receive the information, resources, and networks necessary to start and grow successful businesses.
Three courses will be offered:
- FastTrac NewVenture
- FastTrac GrowthVenture
- FastTrac TechVenture
For more information: Program Flyer
back to top 

University of Maryland (UM) Ventures announced today a collaboration between Frank Robb, Ph.D., at the Institute of Marine and Environmental Technology, and Department of Microbiology & Immunology at the University of Maryland School of Medicine, and Rockville, Maryland-based Fina Biosolutions LLC to devise new ways to manufacture a key component used in many vaccines. The international nonprofit organization, PATH, which spurs new innovation with the goal of delivering high-impact, low-cost global health solutions such as lifesaving vaccines, will fund the partnership’s development efforts as part of its pneumococcal vaccine project.
“A new and improved vaccine production method is the goal of this public-private partnership between my lab and Fina Biosolutions,” said Dr. Robb. “It is designed to yield broader availability of an important vaccine component, which is highly effective, but prohibitively expensive for some applications, particularly in the developing world.”
back to top 

Bethesda tops a list of The Most Educated Places in America
Personal finance site NerdWallet Inc. ranked cities based on high school drop out rates and the percentage of the population with associate degrees, bachelor’s degrees, master’s degrees and professional or doctoral degrees.
back to top 

QIAGEN N.V. (NASDAQ: QGEN; Frankfurt Prime Standard: QIA) today announced it has received approval by the U.S. Food and Drug Administration (FDA) to market the therascreen EGFR test as a companion diagnostic to guide the use of Boehringer Ingelheim’s new targeted therapy, GILOTRIFTM (afatinib), for treatment of metastatic NSCLC in patients whose tumors have certain EGFR gene mutations. More than 200,000 new lung cancer cases are diagnosed every year in the United States, with NSCLC accounting for approximately 85% of cases, leading to an estimated 160,000 deaths.
The therascreen EGFR test enables doctors to identify EGFR mutation-positive patients eligible for treatment with GILOTRIFTM (afatinib). The FDA approval of the therascreen EGFR test marks a further milestone in the global expansion of QIAGEN’s Personalized Healthcare franchise – and adds a third FDA-approved or cleared diagnostic kit to run on QIAGEN’s efficient Rotor-Gene Q MDx. Approximately 120,000 metastatic NSCLC patients each year in the U.S. could benefit from testing for EGFR mutations, a total potential testing market of about $35 million, according to QIAGEN estimates.
back to top 

The Partnering Agreements with United Therapeutics 2005-2013 report provides an in-depth insight into the partnering interests and activites of one of the worlds leading biopharma companies.
This report provides all the information you require to better understand United Therapeutics and its partnering interests and activities over the past seven years.
On demand company reports are prepared upon purchase to ensure inclusion of the most up to date deal and company data.
back to top 

Topic: “EIRs, SBIRs, and more with BioHealth Innovation, Inc.”
Presenters:
- Richard Bendis, President and CEO
- Ethan Byler, Director, Innovation Programs
- Todd Chappell, Entrepreneur-in-Residence, NIH-OTT
- Dr. Ken Malone, Entrepreneur-in-Residence, UMD Ventures
- Ram Aiyar, Entrepreneur-in-Residence, NHLBI
BioHealth Innovation, Inc. (BHI) is a regional innovation intermediary that accelerates and facilitates technology transfer and commercialization of market-relevant research in federal labs, universities, and biohealth companies in the Region. It is a private-public partnership that connects the Region’s innovation assets to provide integrated technical knowledge, financial means, and entrepreneurial/managerial expertise to turn promise into prosperity for the region while advancing human health.
BHI’s Entrepreneur-in-Residence (EIR) program is designed to be an active partner with research institutions to source, fund, and grow high-potential, early-stage products through project-focused companies. The entrepreneurs in the program support the formation of new companies based upon innovative discoveries in the areas of drugs, vaccines, therapeutics, diagnostics, and medical devices from the intramural research programs at the NIH and Food and Drug Administration (FDA), as well as from universities and businesses.
BHI’s Commercial Relevance Program (CRP) offers biohealth companies support in preparing applications for federal funding inclusive of SBIRs, STTRs, and other federal government awards. Companies submit their federal funding concepts and receive pre-proposal feedback to help troubleshoot and strengthen your application. Further support from professional consultants and service providers is available to assist in improving your application.
BHI recently published the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide serves as a compendium of resources to biohealth innovators and entrepreneurs working to start and grow new companies and technologies in the region.
back to top 

Lower overall costs of occupancy — coupled with academic resources and an educated work force — have made clusters outside the nation’s largest metro areas more attractive to multinational pharmaceutical companies focusing on right-sizing and R&D productivity. In the Midwest, Chicago remains an emerging cluster, while Minneapolis is holding steady as a top-10 cluster for the second year in a row. JLL’s second annual life sciences cluster report ranks top-10 cities for life sciences companies in 2013.
back to top 

The head of the nation’s medical research agency and leaders of Johns Hopkins hospital and medical school warned Monday that progress in fighting diseases could be slowed, jobs lost and scientists driven overseas unless across-the-board federal funding cuts are reversed.
Dr. Francis Collins, director of the National Institutes of Health, joined Sen. Barbara A. Mikulski, Hopkins executives and a stroke survivor at Hopkins’ Children’s Center to appeal for restoration of $1.5 billion in NIH funding cuts as part of the budget “sequester” approved last winter by Congress.
back to top 

Renee M. Winsky of Davidsonville has been named president and CEO of Leadership Maryland, a statewide leadership development program.
She replaces Nancy Minieri, who founded the organization in 1992. Minieri announced in March that she will retire at the end of this year.
Winsky is a 2005 graduate of Leadership Maryland. In the yearlong program, participants learn about problems and opportunities facing the state. More than 900 people have taken the course, including elected officials, business executives, educators and nonprofit directors.
back to top 

Innovation is a vague word and is too overused today, in the words of Bryan Sivak.
That was kind of a bold statement to make at a conference focused on, well, innovation.
In his keynote address at CONVERGE, the chief technology officer for the U.S. Department of Health and Human Services said the department’s philosophy is that innovation happens as a direct result of the freedom to experiment, a much more descriptive and meaningful phrase.
back to top 

At the funding panel discussion Wednesday at CONVERGE, a serial entrepreneur asked about crowdfunding.
First we got the stock answer of “don’t see how it’s possible for healthcare companies.” Then the conversation turned to cap tables.
Elliott Menschik of Dreamit Ventures said that having 50-60 people on a cap table is becoming normal.
back to top 

Microryza.com (not the catchiest of names) is a crowdfunding platform for research that raises money over the Internet from individuals who are willing to donate small amounts to fund a specific project. The average donation according to Microryza is $92.
In return for a 5 percent cut of funds raised and a 3 percent credit card processing fee, Microryza provides researchers access to a website where they can solicit money from the public to fund their research. Crowdfunding is typically an all-or-nothing deal, where donors only have to pay their pledged support if the project is fully funded within a defined period of time.
back to top 

CMS has proposed a policy change that would expand payments for telehealth services under the Medicare Physician Fee Schedule for 2014, Modern Physician reports.
Details of the Proposal
The proposed change would affect locations identified as rural “health professional shortage areas.”
back to top 
Indiana University scientists have transformed mouse embryonic stem cells into key structures of the inner ear. The discovery provides new insights into the sensory organ’s developmental process and sets the stage for laboratory models of disease, drug discovery and potential treatments for hearing loss and balance disorders.
A research team led by Eri Hashino, Ph.D., Ruth C. Holton Professor of Otolaryngology at Indiana University School of Medicine, reported that by using a three-dimensional cell culture method, they were able to coax stem cells to develop into inner-ear sensory epithelia — containing hair cells, supporting cells and neurons — that detect sound, head movements and gravity. The research was reportedly online Wednesday in the journal Nature.
back to top 

This week, Healthbox launched a new accelerator program in partnership with BlueCross BlueShield in Nashville, Tennessee. Other Healthbox locations include Chicago, Boston, London and most recently, Jacksonville, Florida. Healthbox typically partners with the local Blue health plan in each of its locations.
Nashville, home to 31 hospitals and more than 250 healthcare companies is “the Silicon Valley of healthcare,” according to Healthbox Founder and CEO Nina Nashif. In 2011, Nashville’s venture capital community invested $104 million in healthcare services and HIT startups. The accelerator also partnered with the Nashville Healthcare Council, an initiative of the Nashville Area Chamber of Commerce to establish Nashville’s position as the nation’s healthcare industry capital. According to the Nashville Healthcare Council, in 2008, one in eight Nashville workers were employed by a healthcare provider.
back to top 

The biotech IPO window is officially open.
Eleven second-quarter biotech initial public offerings totaling $1 billion — the strongest quarterly biotech market for venture-backed IPOs since third-quarter 2000 — fueled an overall surge in IPOs, according to the National Venture Capital Association and news and data company Thomson Reuters.
back to top 

Even as the White House has backed off on a few of its deadlines for administering the Affordable Care Act, the Obama Administration is staying the course on its efforts to transition paper files to electronic health records in doctor’s offices.
President Barack Obama met with several chief executives of health-care technology companies, government leaders and nonprofit public-service organizations on Monday for a conversation on how technology, big data and innovation can be used to bring down the costs and improve the quality of health care in the U.S., according to a statement from the White House.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
Wednesday, August 14, 2013, 03:30pm – 05:00pm
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc., (BHI) a non-profit organization which strives to facilitate the development of commercially viable health IT products and companies by connecting market relevant research assets to appropriate funding, management and markets, is seeking interns as part of the BHI Internship Program.
POSITION DESCRIPTION – BHI Intern
BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, is seeking highly motivated and dynamic individuals to fill internship positions at the Rockville, MD headquarters. The BHI Internship Program offers essential experience for IT, Business, and Biohealth students in the region. By offering a variety of projects and responsibilities to students, BHI aims to prepare interns for professional careers in the business, life sciences, healthcare and IT industries.
back to top 

Montgomery County’s top biotechnology firms will open their doors and their state-of-the-art laboratories next week to local middle school students interested in the life sciences. In a partnership with MdBio Foundation, Inc., and Montgomery College, preeminent local scientists and executives from MedImmune, Qiagen and Emergent BioSolutions will share their expertise and career stories with students from the Young Science Explorers Program (YSEP), a weeklong science workshop for rising seventh and eighth grade students.
Dressed in lab coats and safety glasses, students will get once in a lifetime exposure to local companies and scientists on the leading edge of medical breakthroughs. Topics will range from microbiology and vaccine research to lab safety and potential career paths for science students.
back to top 

Registration for the fall semester of Technology Transfer Classes at the FAES Graduate School at NIH is now open. For the semester beginning on September 9th there will be 12 courses offered in the technology transfer program:
· TECH 506 – Research Commercialization Webinar Course: The Essentials
· TECH 513A Introduction to Technology Transfer — Issues and Processes (given by the Technology Transfer Society)
· TECH 521 – Tools for Technology Transfer
· TECH 565 – Biomedical Business Development for Scientists
· TECH 567 – International Strategic Partnering and Business Development
· TECH 579 – Introduction to Negotiation
· TECH 575 – Business Finance & Accounting Principles for Scientists
· TECH 583 – Patent Research for Non-Legal Practioners
· TECH 587 – Strategic Consulting for Tech Companies
· TECH 607 – Capstone Course in Technology Transfer
· PHAR 328 – FDA Perspective on Drug Development
· PHAR 500 – Principles of Clinical Pharmacology
More details can be found in the new 2013 course catalog (www.faes.org) with enrollment open to the general community. All classes are part of the “Advanced Studies in Technology Transfer” certificate program and can be fully transferred as a block into various MBA & MS degree programs at the University of Maryland University College (UMUC) Graduate School.
There will also be an open house for the FAES Graduate School on Tuesday, August 20th from 4-7pm at the new FAES Classroom & Bookstore complex in Building 10.
back to top 

The INNo program trains research scientists in the entrepreneurial skills needed to bring technology inventions and services to the healthcare market.
Participants in the INNo program learn to:
- Identify and evaluate the commercial potential of intellectual property
- Understand the business fundamentals related to technology start-ups
- Create a value proposition and business concept for a new product, platform, or service
- Articulate investment opportunities persuasively to potential investors and partners
- Develop a network of resources in the Maryland entrepreneurial community
back to top 
Science Applications International Corporation (SAIC) SAI +0.46% , Maryland’s Department of Business and Economic Development (DBED), and the University of Maryland, Baltimore County (UMBC) announced the third annual 2013 statewide cyber competition, the Maryland Cyber Challenge(TM), will be held October 8 through October 9 at the Baltimore Convention Center in Baltimore, Maryland. Registration is now open for aspiring cyber warriors from around the nation to compete at the Maryland event, located in the growing epicenter for the cybersecurity industry.
The Maryland Cyber Challenge(TM) is designed to attract more students and young professionals to pursue careers in cybersecurity and is held in conjunction with the CyberMaryland2013 Conference and Cyber Hall of Fame. It is the premier statewide cyber competition showcasing today’s students and tomorrow’s technologists with three levels of competition: high school, college and professional. Teams will have the opportunity to develop and improve their cybersecurity skills in a real-world environment. Founders of the event include SAIC, UMBC, DBED, the National Cyber Security Alliance (NCSA), and the Tech Council of Maryland (TCM).
back to top 

Due to the many changes that will result from the SBIR/STTR Reauthorization Act, NIH has set up a new website to keep the small business research community informed.
Applicants and grantees interested in the SBIR program can visit the website as well as the SBIR listserv and Twitter feed for news as the implementation plan rolls out.
back to top 

1776, the D.C. startup hub that launched earlier this year with District funding, plans to pull together a seed fund that will allow it to make equity investments in early-stage tech companies.
The creation of a fund would turn 1776 into a bona fide “accelerator.” The 1133 15th St. NW space, run by Startup DC’s Evan Burfield and Donna Harris, is now primarily a co-working operation (some say incubator), with additional programs layered on top of it, like the recently announced Challenge Cup international startup tournament.
back to top 
I t’s good to have friends in high places. Especially friends who can leverage more than 30 years of experience in technology and business to nurture and foster your startup seed. t’s good to have friends in high places. Especially friends who can leverage more than 30 years of experience in technology and business to nurture and foster your startup seed.
1776, a new startup incubator based in Washington, D.C., just announced a brand-new partnership with Microsoft, in which leaders from the company will collaborate with startups to guide them from a technical and business standpoint.
“[Microsoft] will help startups to leverage platforms upon which they can build innovative apps with the ability to scale rapidly and efficiently,” says 1776 co-founder Donna Harris. “Through 1776, they’re also providing mentorships for architects who can ensure that startups are maximizing the potential of these tools and platforms.”
back to top 

As investments in life science companies have dipped over the past few years, some investors have scaled back investments or walked away from the sector all together. But based on a tally by investment research firm PitchBook in its 2013 Venture Capital Healthcare Report, several firms have made 10 or more investments in pharmaceutical, medical device or health IT companies in the last year and a half.
back to top 

Creating a new medical device is not an inexpensive exercise. There are development costs, clinical trial expenses, and the lengthy process of getting a piece of new equipment approved by the Food and Drug Administration.
Those hurdles are a leading reason why most medical innovations come out of universities or established research firms.
back to top 

Funding for digital health startups is continuing to grow at a steady pace, but is beginning to stabilize.
Health accelerator Rock Health’s midyear report, released today, found that digital health funding for the first half of 2013 represents 11 percent growth in investment and 24 percent growth in deals year over year. In short, it’s still growing, but not as fast as in previous years.
back to top 

It’s been a slow year for startup fundraising—the investments doled out by venture firms in the first quarter dropped 6 percent compared with the same period of 2012. But at least a couple of sectors have been defying that trend. One is software, where funding for the first half of 2012 was up 38 percent over 2012 levels, and another is digital health, up 12 percent.
In a report issued yesterday (slides embedded below), San Francisco-based digital health accelerator Rock Health delved into the details behind that 12 percent number.
back to top 

When health IT startups say they’re making a new technology platform for physicians, John Sung Kim has an immediate follow-up question – for what specialty?
That’s a question he learned was critical as he began building and trying to sell the platform developed by his first health startup, DoctorBase. It’s been a series of hits and misses, which Kim isn’t afraid to talk candidly about. In a recent interview, he explained to MedCity News one of the most surprising things he learned when he set out to build a mobile platform that would let doctors, administrators and patients communicate without having to pick up the phone.
back to top 

Five years ago I built a biotechnology innovation index and I have been using it since tracking global biotechnology innovation in Scientific American’s Worldview. It has been a very rewarding project, and I have enjoyed the opportunity to present my research data at international conferences, business schools, and even National Defense University.
Now, Worldview’s editor Mike May has compared my innovation scores with the Venture Capital and Private Equity Country Attractiveness (VCPECA) index. I was quite pleased to see a relatively strong correlation between my innovation index and the VCPECA index.
back to top 

We knew that biotech companies were lighting up the stock market this year. Today, the National Venture Capital Association has fresh numbers illustrating Wall Street’s love affair with life sciences entrepreneurs—and the corresponding cold shower awaiting their counterparts in the tech sector.
The new report, comparing venture-backed IPOs with merger-and-acquisition deals, shows life sciences companies accounting for 13 of the 21 public stock listings in the second quarter.
back to top 

Revolution Ventures, the early-stage venture capital arm of Steve Case’s Revolution LLC, has filed paperwork with the Securities and Exchange Commission for a planned $150 million fund. Named in the SEC filing are Case and managing partners Tige Savage and David Golden. Revolution Ventures II LP, as it’s named in the filing, follows the formation in 2011of Revolution Growth, which invests in more mature, later-stage companies. Revolution Growth, led by Case, Donn Davis and Ted Leonsis, is operated separately from the venture unit.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

July 10
William E. Hanna, Jr. Innovation Center

July 10
University of Maryland BioPark, Life Sciences Conference Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc., (BHI) a non-profit organization which strives to facilitate the development of commercially viable health IT products and companies by connecting market relevant research assets to appropriate funding, management and markets, is seeking interns as part of the BHI Internship Program.
POSITION DESCRIPTION – BHI Intern
BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, is seeking highly motivated and dynamic individuals to fill internship positions at the Rockville, MD headquarters. The BHI Internship Program offers essential experience for IT, Business, and Biohealth students in the region. By offering a variety of projects and responsibilities to students, BHI aims to prepare interns for professional careers in the business, life sciences, healthcare and IT industries.
back to top 

The INNo program trains research scientists in the entrepreneurial skills needed to bring technology inventions and services to the healthcare market.
Participants in the INNo program learn to:
- Identify and evaluate the commercial potential of intellectual property
- Understand the business fundamentals related to technology start-ups
- Create a value proposition and business concept for a new product, platform, or service
- Articulate investment opportunities persuasively to potential investors and partners
- Develop a network of resources in the Maryland entrepreneurial community
back to top 

Gaithersburg’s largest private employer will add some 110 new jobs to its local and Frederick offices and seeks to take its revenues to unprecedented heights by 2020, a official told the Gaithersburg mayor and City Council Monday.
MedImmune’s Gaithersburg headquarters currently house 2,300 employees, approximately 66 percent of the company’s international jobs, MedImmune Executive Vice President of Operations Andy Skibo said, including the addition of 830 jobs over the past five years.
“Virtually all of MedImmune is practically here in Gaithersburg or just up the road in Frederick,” Skibo said, but the biotech company continues to expand.
back to top 

Johns Hopkins Montgomery County has received the 2013 Visionary Award from the Montgomery County Chamber of Commerce.
Before an audience of hundreds at the Chamber’s Annual Awards Dinner, Campus Executive Elaine Amir thanked the Chamber for the recognition, thanked Montgomery County for having the foresight to establish the campus in 1985 and applauded County Executive Ike Leggett for his encouragement and support.
back to top 

The University of Maryland has named Brian Darmody associate vice president for corporate and foundation relations. In this newly-created role, Darmody is charged with leading essential university-wide efforts to develop strategic partnerships between the University of Maryland and the corporate and foundation community.
“Throughout Brian’s 30-year career with the university, he has proven to be the perfect candidate to lead this new charge,” says UMD Vice President for University Relations Peter Weiler. “His unparalleled ability to develop and nurture mutually beneficial relationships for the university has been integral over the years, and we look forward to the leadership he will bring to this new role.”
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
NOT-EB-13-002: Notice To Extend PAR-10-234 Bioengineering Research Partnerships (BRP) (R01)
The purpose of this notice is to extend PAR-10-234 “Bioengineering Research Partnerships (BRP) (R01),” which supports partnerships for basic, applied, and translational multi-disciplinary research that addresses important biological, clinical or biomedical research problems. The new expiration date is January 8, 2014.
NOT-RM-13-022: Notice of Intent to Publish a Funding Opportunity Announcement for the NIH Health Care Systems Research Collaboratory – Demonstration Projects for Pragmatic Clinical Trials Focusing on Multiple Chronic Conditions (UH2/UH3)
The National Institutes of Health, Office of Strategic Coordination intends to promote a new initiative by publishing a Funding Opportunity Announcement (FOA) to solicit applications for Demonstration Projects for Pragmatic Clinical Trials focusing on the management of multiple chronic conditions, to be conducted as part of the NIH Health Care Systems Research Collaboratory.
Requests for Applications (RFAs):
RFA-CA-13-008: Person-Centered Outcomes Research Resource (U2C)
The purpose of this Funding Opportunity Announcement (FOA) is to support the creation of a research resource infrastructure for the administration of research investigations using person-centered health outcomes, further referred to as the Person-Centered Outcomes Research Resource (PCORR).
RFA-OD-13-010: Tobacco Control Regulatory Research (R21)
The purpose of this FOA is to encourage biomedical, behavioral, and social science research that will inform the development and evaluation of regulations on tobacco product manufacturing, distribution, and marketing.
RFA-OD-13-011: Tobacco Control Regulatory Research (R01)
The purpose of this FOA is to encourage biomedical, behavioral, and social science research that will inform the development and evaluation of regulations on tobacco product manufacturing, distribution, and marketing.
RFA-OD-13-012: Tobacco Control Regulatory Research (R03)
The purpose of this FOA is to encourage biomedical, behavioral, and social science research that will inform the development and evaluation of regulations on tobacco product manufacturing, distribution, and marketing.
back to top 

Governor Martin O’Malley announced today that the State, through the BioMaryland Center, has awarded nearly $1 million to five innovative life sciences companies through its Biotechnology Development Awards program. The companies, which received up to $200,000 each, will use the funding to advance the early detection of Alzheimer’s disease, create a less-invasive treatment for tachycardia patients, enhance animal analgesics, control traumatic bleeds and develop high-quality gluten and allergen-free kosher food products.
“These companies are developing products that are changing the way we feed, fuel and heal our planet and have the potential to impact millions of patients around the world,” said Governor O’Malley. “These awards are critical to ensuring that the life-saving research being done here in Maryland has the opportunity to move to the commercial marketplace.”
back to top 

Health and Human Services (HHS) Deputy Secretary Bill Corr announced today that the Department is seeking innovators and entrepreneurs to apply for the HHSentrepreneurs Program. Launched last year, HHSentrepreneurs connects private sector innovators and entrepreneurs with teams of federal employees working on projects that address some of the biggest challenges in health, health care and human services.
The first individuals hired last October through HHSentrepreneurs are working on critical projects including the Affordable Care Act, health resilience technology, and the nation’s organ transplant system.
“By bringing the best in the public and private sectors together, HHSentrepreneurs is creating an environment in HHS that fosters innovative solutions to new and old challenges,” Deputy Secretary Corr said.
back to top 

Research universities and start-up companies receive funds to further develop technologies in the fields of therapeutic, software, medical, mobile and online technologies
The Maryland Innovation Initiative (MII), which accelerates commercialization and technology transfer from university labs to start-up companies, announced it has awarded $2,960,466 to 29 research projects. Funds were awarded to nine start-up companies and 20 university projects – three of these projects include a partnership between two universities working together on technology development. Awards were given across a variety of industries, including therapeutic, software, medical, mobile and online technologies. MII is administered by the Maryland Technology Development Corporation (TEDCO).
“Maryland has some of the best research universities in the nation and an incredible entrepreneurial spirit, which is evident in the awards granted through the Maryland Innovation Initiative,” said Dominick Murray, Secretary of the Maryland Department of Business and Economic Development. “With a progressive approach to university research and technology development, Maryland is well positioned to build on our history of discovery, innovation and invention.”
back to top 

Sources inform “Globes” that AstraZeneca plc (NYSE; LSE; OMX: AZN) has teamed with Israeli real estate company Minrav Holdings Ltd. (TASE: MNRV) to bid in the Office of the Chief Scientist’s biotechnology incubator tender. AstraZeneca will handle the consortium’s professional side, and Minrav will be responsible for financing.
The AstraZeneca-Minrav consortium is bidding against a consortium of OrbiMed Israel and the venture capital arms of Johnson & Johnson (NYSE: JNJ) and Japan’s Takeda Pharmaceuticals Co. Ltd. (TSE: 4502).
back to top 
New Enterprise Associates has increased its focus on companies developing drugs for rare disease, an effort that’s paying off. Portfolio company Prosensa Holding BV, which it backed last year, has just gone public on Nasdaq. The company develops therapies for rare conditions such as Duchenne muscular dystrophy, myotonic dystrophy and Huntington’s disease.
back to top 

UMBC recently earned accolades in three college ranking reports.
The Online College Database recognized the high salaries of UMBC graduates in its list of most affordable colleges. The website used data from the ”2012-2013 PayScale College Salary Report” to rank UMBC as having the second-highest post-graduation starting salary of colleges in Maryland with annual tuition under $20,000. According to the report, the average starting salary of a UMBC graduate is $50,300.
back to top 

Taking the helm of EFPIA, Viehbacher says R&D and innovation are the keys to rescuing Europe from its economic slump, but more harmonization across the pharmaceutical sector will be needed too.
back to top 

Baltimore City’s Emerging Technology Centers (ETC) is pleased to announce that two of its client companies were recently honored at the 13th Annual 2013 Maryland Incubator Company of the Year (ICOY) Awards. Curiosityville was chosen “Best Education Technology Company” and ADASHI was chosen “Best New Incubator Company” by the judging panel of venture capitalists, government officials and business leaders.
“The Curiosityville team is thrilled to have been selected for this award,” said Susan Magsamen, CEO of Curiosityville. “Part of the credit must go to our affiliation with the ETC their assistance has helped allow us to focus on building the business.”
back to top 

“Research for research’s sake” is not a refrain you’ll hear from Johns Hopkins Whiting School of Engineering Dean Nicholas Jones.
Universities have been working on increasing the amount of their research being used for commercially available products and services, but in Maryland the process has been somewhat slow. While Hopkins is the most highly funded university by the National Institutes of Health, it lags behind its peers in terms of patents, new companies and other measures of commercialization.
back to top 

This Funding Opportunity Announcement (FOA) invites Small Business Innovation Research (SBIR) grant applications from small business concerns (SBCs) that propose to implement investigator-initiated clinical trials related to the research mission of the NIAID. This program will utilize the cooperative agreement mechanism (U44) to enable support for hypothesis-driven, milestone-driven clinical trials. Although clinical trials not considered high-risk may be proposed, this program encourages high-risk clinical studies. High-risk does not imply human subject or patient risk, but rather defines a study that contains one or more of the following unique features: involves non-routine interventions, administration of an unlicensed product, or administration of a licensed product for an unapproved indication. Mechanistic studies are also encouraged and can be proposed under this program. However, not more than one clinical trial should be proposed within each grant application. The NIAID has a robust infrastructure for conducting clinical studies that includes independently managed resources provided through grants and contracts, as well as resources that are integrated within existing NIAID-supported clinical trial networks. Proposed clinical trials may use NIAIDs independent infrastructure for clinical studies, however, support will not be provided for studies that propose to use dedicated resources that are part of a NIAID-supported clinical trial network. A Commercialization Plan must be included that details plans for promoting further commercialization of the intervention/product/technology to be derived from or associated with the proposed clinical trial, including plans for promoting and establishing partnerships between the SBIR Phase II awardee and third-party investors and/or strategic partners.
back to top 

Startup Maryland (www.startupmd.org), an initiative of the UpGlobal consortium (www.up.co), today announced that UNDER ARMOUR® Founder and CEO Kevin Plank will participate as the first instructor for Raise Your Game™.
Raise Your Game is Startup Maryland’s bootcamp initiative developed to provide the entrepreneurial community with a structured educational program and to help startup CEOs and founders understand and employ the building blocks of strong startups and startup communities. The new twist for this bootcamp is that the sessions will be proctored/taught by experienced (often serial) entrepreneurs who are very well-recognized and respected.
back to top 

Ernst & Young unveiled its Entrepreneur of the Year Maryland winners on Wednesday night to a packed ballroom at the Marriott Waterfront Hotel in Baltimore, with honors going to former Advertising.com CEO Scott Ferber, the entire Kelly clan and longtime Living Classrooms head James Piper Bond.
A total of 10 awards were given out during a black-tie affair. The awards program recognizes high-growth entrepreneurs who demonstrate excellence and success in such areas as innovation, financial performance and personal commitment to their businesses and communities. The finalists and winners were selected by a panel of independent judges.
back to top 

MyBodyCount® (MBC), a health and wellness platform that enables individuals to track their lifestyle-based health risk, today introduced the first-ever clinical health score available to the public. The MBC Health Score was developed using actuarial science working in conjunction with Dr. Hunter Young, Assistant Professor of Medicine and Epidemiology at The Johns Hopkins University School of Medicine (JHUSOM) and Dr. Dhananjay Vaidya, Associate Professor of Medicine at JHUSOM.
The score is based on a panel of biomarkers, referred to as the BodyCount8™, that are predictors of the risk of health events and conditions related to heart, kidney and lung diseases and diabetes. The biomarkers can be affected by modifying behaviors including: eating, exercising, smoking and medication adherence. The score enables consumers to understand their lifestyle-based risk relative to their age group and gender.
back to top 

A new report on sea level rise recommends that the State of Maryland should plan for a rise in sea level of as much as 2 feet by 2050. Led by the University of Maryland Center for Environmental Science, the report was prepared by a panel of scientific experts in response to Governor Martin O’Malley’s Executive Order on Climate Change and “Coast Smart” Construction. The projections are based on an assessment of the latest climate change science and federal guidelines.
“The State of Maryland is committed to taking the necessary actions to adapt to the rising sea and guard against the impacts of extreme storms,” said Governor Martin O’Malley. “In doing so, we must stay abreast of the latest climate science to ensure that we have a sound understanding of our vulnerability and are making informed decisions about how best to protect our land, infrastructure, and most importantly, the citizens of Maryland.”
back to top 

Venture capital-backed initial public offerings more than doubled during the second quarter from the previous quarter and rose 90% from a year earlier, with 21 companies raising a combined $2.2 billion during their stock-market debuts, driven by the highest number of biotech venture-backed IPOs in nearly 13 years, according to Thomson Reuters Corp. (TRI, TRI.T) and the National Venture Capital Association.
During the quarter, 13 of the offerings were in the life-sciences sector, representing 62% of the total. Biotech offerings, at 11 deals, marked the highest level since the third quarter of 2000, when 13 companies went public.
back to top 

The venture capital industry is getting rightsized, with less capital raised and deployed, smaller funds, fewer active venture capital firms, and more regulation. The exit climate has picked up, but is still not at the level required. And valuations are overall more rational, with some exceptions at the later stages or in consumer-facing momentum companies.
However, with the confluence of not one but four big market drivers (discussed below), and the rise of a new technology cycle, we think this is still a great time to be a venture capitalist or entrepreneur.
back to top 
I ntegrated BioTherapeutics (IBT) and Stanford University have been jointly awarded a $300,000 Small Business Technology Transfer (STTR) grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. The goal of the grant is to develop a highly effective immunotherapeutic to prevent Hepatitis C Virus (HCV) reinfection in liver transplant patients based on antibodies that limit the ability of the virus to escape treatment via mutations. ntegrated BioTherapeutics (IBT) and Stanford University have been jointly awarded a $300,000 Small Business Technology Transfer (STTR) grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. The goal of the grant is to develop a highly effective immunotherapeutic to prevent Hepatitis C Virus (HCV) reinfection in liver transplant patients based on antibodies that limit the ability of the virus to escape treatment via mutations.
Up to 170 million people worldwide are chronically infected with HCV, putting infected individuals at significant risk for cirrhosis, liver failure, and liver cancer. Chronic infection is poorly controlled by current antiviral treatments though there is new optimism with two recent FDA-approved direct acting antivirals, telaprevir and boceprevir. These drugs, however, are not recommended in the transplant setting due to likely adverse drug-drug interactions.
back to top 

A commercialization program to match up provider, payer and pharma technology needs with willing and able healthcare startups has announced its 10 finalists. Each will receive $100,000 tied to meeting certain performance milestones. They have three to six months to work with the healthcare group they’re matched with, depending on the complexity of the program.
PILOT Health Tech NYC, developed by the New York City Economic Development Corporation and Health 2.0, is holding its demo day today at Blueprint Health’s NYC digs. The program is also supported by StartUp Health.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

As our next receipt date is approximately 6 weeks away, we wanted to send a reminder to all new (and returning) HHS applicants about the required registrations needed before you can submit your SBIR/STTR grant to grants.gov. This includes the new requirement to register with the SBA Company Registry. It is never too early to start the registration process, even if you are thinking about submitting for December 5th or beyond, but especially for August 5th!
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the SF 424 (R&R) Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. The NIH Policy on Late Submission of Grant Applications states that failure to complete registrations in advance of a due date is not a valid reason for a late submission.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
Request for Information:
- Request for Information (RFI): Input on Administration of the NIH-Industry Pilot Program Discovering
The goal of this Request for Information (RFI) is to collect feedback from the biomedical research community, pharmaceutical companies, and other members of the public about the “NIH-Industry Pilot Program: Discovering New Therapeutic Uses for Existing Molecules” initiative and the application process. NCATS is interested in feedback from researchers and institutions that submitted an application, considered submitting an application but did not, and/or would be interested in submitting an application in the future. NCATS is also interested in feedback from existing and potential pharmaceutical partners.
back to top 

Higher education is becoming big business as more students head off to college, but they’re also about developing businesses, and the state is turning research and development into new business ventures.
Maryland leads the nation in how much it spends on research but the University System of Maryland — which includes 13 colleges and universities — is trying to take research to a new level by creating more small businesses off campus. The goal so far is to try and create more than 300 new businesses over the next decade.
back to top 

Montgomery College has become a certified affiliate of the Kauffman Foundation and its FastTrac series of courses intended to support future and current business owners before, during, and after the start-up process.
The course schedule is now available in the attachment below.
FastTrac courses provide entrepreneurs the information, resources, and networks necessary to start and grow successful businesses.
back to top 

Johns Hopkins University is seeing strong interest for the university’s first business accelerator — a faster than expected response from what director John Fini had projected.
“It’s like we’re tapping into something,” said Fini, who also leads the Office of Intellectual Property and Technology Commercialization on the university’s Homewood campus. “The palate was there. They just didn’t have an outlet.”
The accelerator, called FastForward, opened in January to Hopkins faculty members and students interested in pursuing business ideas with their research. The university is holding an official grand opening for the accelerator June 27.
back to top 

Silicon Valley has long been the epicenter of venture capital-financed high-technology, but a new report shows D.C. is climbing the ranks of best metropolitan areas for venture capital, according to The Atlantic Cities.
Martin Prosperity Institute’s figures for venture capital in 2012 show than the San Francisco-Oakland area has in fact overtaken it as the nation’s leading center for venture capital, with investments reaching more than $6.8 million.
back to top 

Last fall, the Federal Laboratory Consortium for Technology Transfer introduced an improved Available Technologies search tool that made it faster and easier to search for federal laboratory inventions available transfer to business partners. This tool reduces the time, effort and guesswork in finding opportunities that meet industry’s needs. Instead of sifting through agency or lab records, users can now do single keyword searches for available technologies. Since the search is based on Google search technologies, users can utilize standard Boolean search engine language to perform their searches. Searches return a powerful set of data, including:
- description of the invention
- application and benefits
- current development and patent status
- name of inventor
- federal laboratory contact person who will facilitate the technology transfer opportunity.
back to top 

Johns Hopkins student-built devices—a blood clot detection system and a concealable, hands-free breast pump—have won two of the top three awards in a national contest that recognizes innovative biomedical engineering designs that have high commercial potential and social impact.
The honors were announced June 19 in Philadelphia by the National Collegiate Inventors and Innovators Alliance (NCIIA), as it concluded its annual Biomedical Engineering Innovations, Design, and Entrepreneurship Awards (BMEidea) competition. Johns Hopkins student teams previously earned first-place in this competition in 2012, 2010 and 2007.
back to top 

A tiny, tin-coated piece of wood could one day be used as a high capacity and environmentally benign battery.
A team of researchers at the University of Maryland constructed a nano-scale, sodium-ion battery from a sliver of wood more than 1,000 times thinner than paper.
“The inspiration behind the idea comes from the trees,” said Liangbing Hu, an assistant professor of materials science, in a press release. “Wood fibers that make up a tree once held mineral-rich water, and so are ideal for storing liquid electrolytes, making them not only the base but an active part of the battery.”
back to top 

iVantage Health Analytics, a leading provider of health care informatics and business analytics solutions that transform complex data into actionable business intelligence, announced it has raised $10 million in growth equity from Great Point Partners (GPP). The equity investment will fund expanded sales and marketing efforts, additional investment in technology infrastructure and personnel expansion.
SC&H Capital, an investment banking and advisory firm focused on middle market and growth companies, served as the sole placement agent to iVantage Health Analytics in connection with the transaction.
back to top 
The NIH has been faced with considerable difficulties as of late in terms of finding the required means to continue moving science forward at the early stage. However, the group recently announced a commitment of $12.7 million to a novel project – funding further research on assets that have been cast-off by big pharma in key indication areas that represent a significant unmet medical need (e.g. Alzheimers, Duchenne, etc.). The initiative has been fittingly named Discovering New Therapeutic Uses for Existing Molecules, and it may be a groundbreaking solution to several problems facing drug development today. These include reducing time to market, alleviating early stage investment risk, and creating even more incentive for research scientists to orient themselves towards commercialization of research.
back to top 

The Robert H. Smith School of Business at the University of Maryland will offer an online MBA program beginning in January 2014. Designed to accommodate working professionals, the flexible online program allows students to earn an MBA degree largely on their own time with minimal on-campus requirements. The program courses are taught by the same top faculty and adhere to the academic rigor of the Smith School’s other top MBA programs.
“This is a major step forward for the university and for business professionals who need flexible access to academic excellence,” said UMD President Wallace Loh. “The Smith School is combining a state-of-the-art online platform with the academic rigor that makes it a leader. University-wide we are exploring how best to use technology-based learning, and this is an excellent model.”
back to top 

The Department of Defense last week announced the establishment of the world’s first brain tissue repository on the Walter Reed Campus to help researchers better understand traumatic brain injury (TBI).
TBI is common among veterans of the wars in Afghanistan and Iraq, which led to the opening of The Center for Neuroscience and Regenerative Medicine Brain Tissue Repository for Traumatic Brain Injury at the Uniformed Services University on the campus.
back to top 
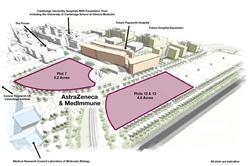
“Don’t write that we have a cure for cancer tomorrow,” says Pascal Soriot, chief executive of AstraZeneca, “but there are some spectacular results.”
Mr Soriot was at MedImmune on Granta Park, AstraZeneca’s current toe-hold on the city and on the day that the pharma company announced its chosen site – Cambridge Biomedical Campus at Addenbrooke’s – to relocate its global HQ and UK R&D activities.
back to top 

The search for the artificial pancreas continues, and the Juvenile Diabetes Research Foundation (JDRF) and Becton Dickinson ($BDX) have joined forces to develop an insulin-delivery system paired with glucose monitoring for people with Type 1 diabetes.
The foundation and Becton Dickinson have agreed to a three-year partnership, an extension of existing collaborations between the two, with a focus on Type 1 diabetes, according to a release. The device, long sought-after in the industry, would mimic the functions of a human pancreas to release insulin when blood sugar levels indicate it is needed by combining the pump with a glucose-monitoring system. This minimizes the potential for human error, as the standard of care currently requires a pump with a catheter and patient-controlled glucose monitoring.
back to top 
Hard to understand, slow to exit and more than enough risk to go around.
Not many investors are brave and smart enough to fund startups working to get new pharmaceuticals, medical devices and therapeutics on the market. To highlight this breed of investors, and to give healthcare entrepreneurs a way to find them, we have compiled a list of venture capital firms with a track record of healthcare investing.
back to top 

Following is a list of 10 alliances announced in recent years, mostly by pharma and biotech giants with venture capital funds, ranked by total size of fund in which the biopharma(s) invested. Alliances are listed by their partners; their purpose; the role of their partners; the financial contributions of their partners, where disclosed; rights and/or options on drugs resulting from alliance activity, again where disclosed; and the date the alliance was announced. An additional two alliances did not disclose size of total investment, and therefore are included in the list without a ranking.
Significantly, five of the 12 listed alliances were formed during 2013, and another five last year, reflecting the industry’s increasing view that the alliances will offer a more efficient way of developing new drugs by requiring much less than the billions long spent up-front by biopharmas on internal R&D. While the alliances require much less capital from industry, it remains to be seen whether R&D activity will increase, and more new drugs win approval and reach the market, to justify the reduced investment.
back to top 

BECTON, Dickinson and Co.’s announcement that it was about to roll out a new, easy-to-use, disposable pen injector called Vystra hardly caused a stir in October.
Although an executive for the Franklin Lakes, New Jersey-based medical technology maker said the injector, unveiled at a Las Vegas convention, would introduce “a new level of flexibility for drug manufacturers,” the announcement made few ripples outside the industry.
back to top 

This is the season of inaugurations and internships, with the class of 2014 college grads starting a new chapter in life in a recovering yet still challenging job market, and with students beginning a summer of exploring what to do beyond school lives. Whether it’s a full-time job or an internship, the experience is as much about learning the knowledge and skills as it is about self discovery.
I recently met a white, middle-aged American, who is highly fluent in Mandarin and successful running a center whose work requires fluency in East Asian cultures and languages. With an impressive list of life experiences under his belt, he was obviously happy with his life and career. When asked how he got to this point in life, he insisted it was pure “dumb luck” because he couldn’t have foreseen the many opportunities related to his interests when he was a young man. I can relate to that. I am more confident and content with my work life than ever before, having finally found my ways of relating and contributing to the world around me. I wish I could say this was all by design, when in fact for the first decade in this country my life was defined by heartbreaks and headaches. As a liberal arts major and a generalist with broad interests, I was not as readily employable as those with technical such as IT and engineering, so I struggled for a long time to find my footing.
back to top 

UK-based healthcare company GlaxoSmithKline (GSK) has announced an investment of €17.5m ($23.5m) in a French venture firm to develop new drugs that have the potential to target rare diseases in Europe.
The Kurma Biofund II fund is led by Paris-based venture capital firm Kurma Life Science Partners (KLS) and is supported by other investors namely, CDC Entreprises, Idinvest Partners and New Enterprise Associates.
back to top 

BlueCross BlueShield of Louisiana and Blue Cross Blue Shield of Massachusetts will provide online healthcare to their members with technology from American Well.
Blue Cross and Blue Shield of Louisiana plans to leverage American Well’s telehealth platform as an added feature of its new Quality Blue Primary Care population health and quality improvement program, and will create multiple avenues to use this technology in other programs.
back to top 

Venture capital firms—and the high-tech startups they support—historically concentrated in suburban office parks, such as Silicon Valley and North Carolina’s Research Triangle.
Richard Florida, co-founder and editor of The Atlantic Cities and director of the Martin Prosperity Institute, has crunched the available data and found (along with some other studies) a shift underway within the venture capital community away from the suburbs and towards urban areas.
back to top 

Business, civic, and political leaders in St. Louis are coming together to raise $100 million in private funds over the next five years to support the Regional Entrepreneurial Initiative, a new effort aimed at helping emerging regional businesses grow and thrive. The project was launched with funding from the federal government and will draw on several ongoing fundraising initiatives in the community. About 80 percent of the funds will be used to provide capital support for startup businesses, with the remaining 20 percent directed toward entrepreneurial support and mentoring, according to the St. Louis Beacon.
In April 2012, the city and county of St. Louis invited stakeholders to develop a regional economic development strategy that would guide efforts to grow and retain high-growth startups. The St. Louis Regional Entrepreneurship Initative Report received financial assistance from the Economic Development Administration (EDA), the state of Missouri, and the St. Louis County Economic Council. The findings of the report suggest that the St. Louis region has a growing cluster of resources to support entrepreneurship, but that they are not of uniform quality and not distributed evenly across sectors. For example, the region’s bioscience sector is relatively well-supported through the efforts of such organizations at BioSTL, BioGenerator and the Helix Center Biotech Incubator. Information technology companies, however, have few local organizations and programs to rely on for support. Resources for the agricultural, energy and advanced manufacturing sectors remain scarce and uncoordinated.
back to top 

The America Invents Act (AIA), also known as the “patent reform bill,” is designed to modernize the U.S. patent system. One provision of the new bill, that went into effect in March, converts the United States from first-to-invent to a first-inventor-to-file system. You’ve had a few months to get used to the new law, but if you’re still wondering how it could affect biotech and medical-device products you invent, you’re not alone.
The new first-inventor-to-file patent law means that the first person or company to file a patent application on an invention has the right to pursue the patent on it. While this is new in the U.S., it’s the system that’s already being used in every other country in the world. So now the United States should be in synch with the rest of the world, right? Well, not quite.
back to top 
Boston Children’s Hospital stirred up some buzz this week when it said its researchers had made a breakthrough that could change the face of diabetes treatment.
On its Vector blog, the hospital called attention to a study published earlier this year in the journal Diabetes that identified a certain pathway in the body as the cause of type 1 diabetes. A team led by Dr. Paolo Fiorina from the hospital’s nephrology department studied hundreds of pathways in animals with diabetes and isolated one, ATP/P2X7R, as a trigger of T-cell attacks on the pancreas that inhibit its ability to produce insulin.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation is working to assist relevant SBIR projects through its Commercial Relevance Program for the August 5th, 2013 NIH SBIR Deadline.
If your company is planning a submission for the August 5 deadline, BioHealth Innovation can assist with your submission. Through the BHI Commercial Relevance Program for SBIR/STTR projects select companies submit their federal funding concepts and receive pre-proposal feedback to help strengthen your application. Further support from BHI’s network of professional consultants and service providers is available to assist in improving your application. If you’re are planning a submission for the August 5 deadline contact Ethan Byler for details on possible support from BHI.
NIH SBIR Phase I Program
- Companies to complete brief application describing the proposed SBIR project apply by June 18th
- All companies notified of SBIR assistance by June 26th
- Guidance, writing strategy, direction, and review sessions to be schedule to enable you through grant submission
- Feedback and redlined comments on written drafts and proposals materials as well as final submission assistance
back to top 

June 27th, 3:30-5:30pm
Join Nicholas P. Jones, Benjamin T. Rome Dean, Whiting School of Engineering to celebrate the launch of FastForward.
There are a few spots left.
RSVP today!
410-516-8723 / engineering@jhu.edu
Get a preview of the innovative technologies created by Johns Hopkins faculty, postdoctoral fellows, and students in the FastForward accelerator and see examples of entrepreneurship from across the university. Learn how FastForward, a unique innovation system, can increase the probability of a startup’s success.
back to top 

6/28 DEADLINE to reserve your place in line for Maryland’s
FY2014 Biotechnology Investor Incentive Tax Credits.
$10M (up to $1.5M/co.) distributed first come-first served to investors in MD QMBCs*:
QMBC requirements include:
- At least 1 full time employee (cash compensated, not equity) & fewer than 50employees; and
- A lease or ownership oflab or office space in Maryland; and
- $100,000 in capitalization (sources:equity, convertible debt, long term loans, SBIR financing); and
- Evidence of innovative biotechnology research owned by the company and conducted in MD; and
- BIITC participation for 10 or fewer years; or
- For first time applicants: 10 or fewer years in business (12 years if in a FDA regulatory process)
If your firm qualifies for QMBC certification by Maryland’s Department of Business and
Economic Development (DBED), your investors may be able to receive a 50% credit on their
investments in your company.
6/28 is the deadline to apply to receive a pin number providing access to the
7/8/13 electronic line-up for FY 2014 BIITC funds.
For more information regarding the BIITC and other state funding programs and access to the BIITC
investor and company application forms on the DBED web site, check online: www.Bio.Maryland.gov.
*QMBC status will not be determined until after the electronic line-up as applications are being reviewed.
**Additional funds may be available after those requested at the initial electronic line-up on 7/8/2013.
back to top 

University of Maryland, Baltimore campus President Jay A. Perman, M.D., and University of Maryland School of Medicine Dean E. Albert Reece, M.D., Ph.D., M.B.A., wish to announce the establishment of a new center to unite research scientists and physicians across disciplines. The center will employ these interdisciplinary connections to enhance the use of cutting edge medical science such as genomics and personalized medicine to accelerate research discoveries and improve health care outcomes. Participants in the new University of Maryland Center for Health-Related Informatics and Bioimaging (CHIB) will collaborate with computer scientists, engineers, life scientists and others at a similar center at the University of Maryland, College Park campus, together forming a joint center supported by the M-Power Maryland initiative.
University of Maryland School of Medicine Dean E. Albert Reece, M.D., Ph.D., M.B.A., with the concurrence of President Perman, has appointed as co-director of the new center Owen White, Ph.D., Professor of Epidemiology and Public Health and Director of Bioinformatics at the University of Maryland School of Medicine Institute for Genome Sciences.
back to top 
UK pharmaceutical company AstraZeneca plc (LSE:AZN) is the second bidder for the dedicated biotech incubator being set up by the Chief Scientist’s incubator program at the Ministry of the Economy, sources inform “Globes.”
The company will bid for the incubator tender against a consortium of OrbiMed venture capital fund and healthcare giant Johnson & Johnson (NYSE: JNJ).
back to top 

The University of Maryland (UM) BioPark announced today that Ocular Proteomics, LLC (OPL), a startup biotechnology company leveraging a new class of biomarkers found in vitreous fluid of the eye to more accurately diagnose and treat retinal diseases, has recently relocated to Building One of the BioPark from Baltimore County. Led by world-renowned and internationally respected retinal surgeon Bert M. Glaser, M.D., OPL is a spin out of the National Retina Institute. OPL’s move to the BioPark comes on the heels of the company’s $1.2 million dollar Small Business Innovation in Research (SBIR) phase 2 grant from the National Institutes of Health. In its new space, OPL will be conducting the research phase of a multi-center clinical trial, which will include 200 patients with macular degeneration.
Said Jim Hughes, President, Research Park Corporation, University of Maryland Baltimore, “The BioPark has once again attracted an innovative and important start-up to our location. We’re pleased to welcome Dr. Glaser and Ocular Proteomics to our roster of commercial tenants. Dr. Glaser founded Ocular Proteomics to use personalized medicine to drastically change the way blinding diseases are diagnosed and treated.”
back to top 

Irene Pollin, the widow of the late Washington sports team owner Abe Pollin, has donated $10 million to a Johns Hopkins University center for the prevention of heart disease.
Hopkins announced Thursday that Pollin’s gift will support the Ciccarone Center for the Prevention of Heart Disease in the division of cardiology.
back to top 

AstraZeneca (STO:AZN)(LSE:AZN) today announced that MedImmune, its global biologics research and development arm, and NGM Biopharmaceuticals, Inc. have entered into an exclusive agreement to discover, develop and commercialise novel therapeutics from NGM’s enteroendocrine cell (EEC) programme for the treatment of type 2 diabetes and obesity.
EECs represent less than 1% of all gastrointestinal (GI) cells, but produce virtually all of the known GI hormones, including GLP-1. EECs are an underexplored source of novel hormones that could play a major role in the positive and negative regulation of metabolism and glucose homeostasis. NGM has established a proprietary platform capable of isolating and analysing EECs in order to identify novel secreted peptide hormones that are potentially linked to the profound metabolic effects of bariatric surgery and serve as potential targets for the treatment of metabolic diseases.
back to top 

Aside from memory loss and cognitive impairments, often the most difficult aspect of caring for people with dementia is treating their disruptive changes in behavior.
With no reliable medications to treat them and limited information for caregivers regarding alternative therapies, these behavior changes are frequently the source of increased upset, stress and burden to families and often result in nursing home placement.
back to top 
Wednesday, June 19, 2013, 06:00pm – 09:00pm
CoFoundersLab & Society of Physician Entrepreneurs proudly presents an educational and networking forum for entrepreneurs in healthcare and life sciences.
To make the most of your time, please browse CoFoundersLab.com and filter by “Meetup Groups – Society of Physician Entrepreneurs” before attending. If you have not created a profile on CoFoundersLab.com, please note that this is required in order for people to discover you before the event and seek you out during the networking portion of the event.
More Information
back to top 

Many times when I talk with small businesses, they don’t fully understand the difference between a Federal Grant and a Federal Contract. It is important for a small business to completely understand if their government funds are coming from a grant or a contract. The terms and conditions surrounding each have somewhat unique requirements that may have implications on how the business handles the award and, in particular, the accounting related to that award.
The Government defines the difference in fairly easy to understand terms (for the government) at the grants.gov website:
A Grant is an award of financial assistance, the principal purpose of which is to transfer a thing of value from a federal agency to a recipient to carry out a public purpose of support or stimulation authorized by a law of the United States (see 31 U.S.C. 6101(3)). A grant is distinguished from a contract, which is used to acquire property or services for the federal government’s direct benefit or use.
back to top 

The NIH is one of the ‘easier’ federal agencies to apply to because every year it issues an Omnibus Solicitation, requesting investigator- initiated topics. This means that rather than telling you exactly which projects they will fund, the NIH asks you, the investigator, to come up with the ideas.
As long as these ideas are related to Human Health, have the requisite level of Technological Innovation and Commercial Potential, they may be appropriate for SBIR/STTR. However, you still need to do some homework to make sure your idea fits within the research interests of the NIH’s Institutes and Centers. So before you put a lot of work into developing your proposal, there are a few things you can do:
back to top 

On May 31, the Department of Health and Human Services (HHS) reissued its Omnibus Funding Opportunity Announcement (FOA) for the Small Business Innovation Research (SBIR) and the Small Business Technology Transfer (STTR) programs in order to implement venture capital provisions of the SBIR/STTR Reauthorization Act of 2011.
HHS notice NOT-OD-13-071 will allow small business concerns that are majority-owned by multiple venture capital operating companies (VCOCs), hedge funds and/or private equity firms to apply for the NIH SBIR program and compete for up to 25 percent of NIH’s SBIR set-aside in the Omnibus FOA or any other NIH SBIR funding announcement issued hereafter. With this notice, NIH is the first agency to elect to use its authority under Section 5107 of the reauthorization to make awards to majority-owned firms, signaling new and significant opportunities for venture capital in the future.
back to top 

The University Economic Development Association (UEDA) is currently seeking nominations for its annual Awards of Excellence program, which recognizes leading edge university-based economic development initiatives from across the country. The Awards of Excellence Program recognizes higher education institutions and their partners who are transforming their campuses into engines of economic prosperity through creative initiatives in five categories:
- Community Connected Campus: initiatives that promote the physical development of quality connected campuses and their surrounding communities;
- Research and Analysis: initiatives that enhance the capacity of colleges and universities to provide new forms of research and tools for community, economic and workforce development practitioners;
- Leadership and Collaboration: initiatives that support the development of collaborative economic development strategies and the leaders required to implement them;
- Innovation and Entrepreneurship: initiatives designed to support startups, high-growth companies and clusters within a region; and
- Talent Development: initiatives that promote the development of 21st-century skills.
back to top 

It’s a popular sport among startups and the mobile vendor community to figure out what physicians are willing to do on their tablets and smartphones. It has to be said that some of them have come up with some pretty compelling approaches to deepen the relationship between physicians and their mobile devices.
But what is really going on in their practices? In two studies generated from a survey by AmericanEHRPartners of 1,400 with responses from about 696 physicians and 150 allied health professionals has uncovered some interesting information. AmericanEHRPartners was formed in 2010 by formed by Cientis Technologies and the American College of Physicians to help physicians compare and implement electronic health records.
back to top 

Robert Langer shares the experiences and lessons learned through his involvement with more than two dozen biotech startups.
I started my first company in 1987 because I realized it was an effective path for transforming science into life-saving and life-improving inventions. Startup companies provide one means for accomplishing ends that interested me: creating products that have a positive effect on human health. I did this first with a colleague (Box 1), but through the years I have also started many companies with students and postdocs in my MIT lab.
back to top 

In the year following its groundbreaking last June, all aspects of Bioscience Connecticut have moved forward on time and on budget. Of note, the project has created about 500 construction and related jobs on the UConn Health Center campus in its first year, including higher-than-required averages for small business participation and 85 percent of all work going to Connecticut-based contractors. The number of construction jobs will rise significantly over the next three years.
“Bioscience Connecticut was an important first step in positioning Connecticut as a leader in the industry,” said Governor Dannel P. Malloy. “This investment, in conjunction with the new Bioscience Innovation Fund and our other efforts, not only creates thousands of good paying jobs with good benefits, but also highlights the commitment we have to growing this sector of our economy. Our vigorous approach to establishing long-term partnerships between our universities, medical centers, and private secto
back to top 

Emory University has launched a public-private drug development enterprise that will transition scientific discoveries more rapidly and efficiently from university laboratories into the marketplace. The new venture is expected to help address worldwide drug development and commercialization needs.
Drug Innovation Ventures at Emory, LLC (DRIVE) is a not-for-profit company separate from, but wholly owned by Emory.
back to top 

On Tuesday, GE Healthcare announced plans to invest $2 billion over the next five years on the development of software for health systems and applications, Healthcare IT News reports.
To develop the software, the company will work with the GE Software Center of Excellence in San Ramon, Calif., in addition to several other research and development firms across the world (Monegain, Healthcare IT News, 6/12).
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 19
Johns Hopkins University – Montgomery County Campus

June 20-21
Renaissance Washington, DC Downtown Hotel

June 26
Baltimore Marriott Waterfront

June 27
Stieff Silver Building
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Governor Martin O’Malley today announced that Rachel King, co-founder and CEO of GlycoMimetics in Gaithersburg, has been named chair of the Maryland Life Sciences Advisory Board (LSAB). King will replace chair H. Thomas Watkins, former President and Chief Executive Officer of Human Genome Sciences, Inc., who has served on the Board since Governor O’Malley and the Maryland General Assembly created it in 2007. As chair, Watkins led the Board through a strategic planning process that, working closely with Governor O’Malley, resulted in BioMaryland 2020, a 10-year, $1.3 billion strategy for moving Maryland’s life sciences industry forward.
back to top 

The Economic Alliance of Greater Baltimore (EAGB) and Maryland Department of Business of Economic Development (DBED) have announced the creation of Advance Maryland, a business program designed to support growth companies, an integral component in the prosperity and sustainability of local economies. Advance Maryland was established to provide resources targeted to second-stage companies. These companies are growth-oriented and have moved beyond the startup phase. They are at the forefront of job creation and critical to vibrant economies. In contrast to traditional business assistance which focuses on finances, business plans and operational issues,
Advance Maryland addresses strategic growth challenges, from developing new markets and refining business models, to gaining access to competitive intelligence. “Maryland has a plethora of organizations and resources devoted to the startup community, but we are limited when it comes to resources for second stage companies. The statistics show how essential it is to recognize these companies and make the necessary tools available to support their growth,” stated Jen Gunner, COO of EAGB and Co-Program Manager of Advance Maryland. Youreconomy.com states that between 1995 and 2009, second-stage companies represented eleven percent of U.S. establishments, but generated more than thirty six percent of jobs and thirty eight percent of sales.
back to top 

University of Maryland (UM) Ventures and SilcsBio, LLC announced today that SilcsBio has obtained exclusive rights to a technology licensed from the University of Maryland, Baltimore (UMB). UM Ventures is an ambitious joint research commercialization effort of the UMB and the University of Maryland, College Park (UMCP). SilcsBio is a supplier of computer-directed drug discovery software and services.
“The license, which we obtained from UMB, creates the core of our product line,” said Kelli Booth, SilcsBio’s Chief Operating Officer. “It’s great to have a university so supportive of our state’s start-up community.”
back to top 

The Chinese Biopharmaceutical Association, USA (CBA) will host the 18th Annual Conference at the University of Maryland Shady Grove Conference Center on Saturday, June 15th, 2013. The theme of this year’s conference is “Global Partnership in Biopharmaceutics and Translational Medicine;” it will address the critical importance of establishing worldwide collaboration to capture the great opportunity for the advancement of modern medicine and biopharmaceuticals.
The Conference
The Conference includes five sessions:
- Drug Discovery: New Strategies and Platforms
- Translational Genomics and anti-cancer therapy
- Opportunities in New high tech parks in China
- Regulatory Compliances in Biopharmaceuticals
- Collaborative Opportunities and Partnership for U.S.-China Biopharmaceuticals.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Program Announcements (PA):
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

The NHLBI Division of Extramural Research Activities (DERA) is pleased to announce the addition of Dr Lawrence Mahan, as the Director of the Office of Translational Alliances and Coordination (OTAC). Dr. Mahan’s professional experience spans academia, government and industry in both basic and applied biomedical research. Additionally it includes global business and strategic alliance development, strategic planning, technology evaluation, entrepreneurship guidance, and consulting on platform technology development in the life sciences.
Most recently Dr. Mahan served as Director of Innovation and Business Development for Children’s National Medical Center and its research institutes, the Children’s Research Institute and the Sheikh Zayed Institute for Pediatric Surgical Innovation, where he managed intellectual property, strategic business alliance development and the advancement of academic entrepreneurship.
back to top 

The D.C. region is home to some 765,000 jobs that require knowledge in science, technology, engineering and math, representing 27 percent of the overall workforce, according to a report released Monday by the Brookings Institution. Only Silicon Valley ranked higher in percentage of STEM labor.
Greater Washington has consistently ranked near or at the top of the nation in STEM job rankings, owing largely to the federal government and the contracting industry surrounding it, which grew rapidly following the Sept. 11 terrorist attacks. The region also boasts a substantial cluster of commercial tech companies – many of them situated along the Dulles corridor and Interstate 270 – as well as a small but growing software startup scene.
back to top 

Almost a quarter of the jobs in the Baltimore-area are science, technology, engineering and math positions, according to a new report by the Brookings Institution.
A total of 281,730 local STEM jobs account for about 23 percent of workforce in the Baltimore-Towson region. Baltimore ranks eighth out of 100 metropolitan areas for its concentration of STEM jobs in the Metropolitan Policy Program study released Monday by the Washington, D.C., think tank.
back to top 

Maryland is No. 1 — or is it No. 7? On third thought, it might be 41st.
Critics and champions of the Free State’s business climate and tax policy have plenty to argue about most days, and all-over-the-place business climate rankings do little to quell the conflict.
The U.S. Chamber of Commerce last month rated Maryland No. 1 for entrepreneurship and innovation, piling on to an Entrepreneur Magazine ranking calling Maryland the best state in which to start a business.
back to top 

Histogenics Corp., a regenerative medicine company that combines cell therapy and tissue engineering technologies to develop highly innovative products primarily for orthopedic tissue repair, today announced the appointment of Peter Greenleaf to Chief Executive Officer.
Bringing over 20 years of experience in the biotechnology industry to Histogenics, Mr. Greenleaf most recently served as President of MedImmune, the worldwide biologics arm of AstraZeneca. During his tenure, he presided over the expansion of MedImmune’s extensive growth and pipeline and spearheaded industry-leading business development and venture deals. Mr. Greenleaf also served as the President of MedImmune Ventures, overseeing more than $300 million in investments in early stage portfolio companies. Prior to becoming President, Greenleaf led the development of the company’s global marketing and portfolio organizations and managed the broader commercial, corporate development and strategy functions.
back to top 

BioHealth Innovation is working to assist relevant SBIR projects through its Commercial Relevance Program for the August 5th, 2013 NIH SBIR Deadline.
If your company is planning a submission for the August 5 deadline, BioHealth Innovation can assist with your submission. Through the BHI Commercial Relevance Program for SBIR/STTR projects select companies submit their federal funding concepts and receive pre-proposal feedback to help strengthen your application. Further support from BHI’s network of professional consultants and service providers is available to assist in improving your application. If you’re are planning a submission for the August 5 deadline contact Ethan Byler for details on possible support from BHI.
NIH SBIR Phase I Program
- Companies to complete brief application describing the proposed SBIR project apply by June 18th
- All companies notified of SBIR assistance by June 26th
- Guidance, writing strategy, direction, and review sessions to be schedule to enable you through grant submission
- Feedback and redlined comments on written drafts and proposals materials as well as final submission assistance
back to top 

Five to ten years. That’s how long it will be before drug reimbursement in the United States becomes as stringent as in Europe, according to a range of consultants, analysts, and health policy experts with whom I’ve spoken.
This new reimbursement environment – and the expectations leading up to it – is expected to emphasize the value of “profound” innovation, at the expense of less dramatic, incremental innovation.
back to top 
In a first-of-its-kind operation in the United States, a team of doctors at Duke University Hospital helped create a bioengineered blood vessel and transplanted it into the arm of a patient with end-stage kidney disease.
The procedure, the first U.S. clinical trial to test the safety and effectiveness of the bioengineered blood vessel, is a milestone in the field of tissue engineering. The new vein is an off-the-shelf, human cell-based product with no biological properties that would cause organ rejection.
back to top 

NIH has reissued its Small Business Innovation Research (SBIR) Omnibus Grant Solicitation announcement, which states that small businesses that are majority-owned by multiple venture capital operating companies are eligible to apply for (1) these SBIR grants and (2) any other NIH SBIR funding opportunities announced after January 28, 2013. The NIH grant solicitation announcement can be found here.
With this re-issuance, small businesses that are majority-owned by multiple venture capital operating companies (VCOCs), hedge funds and/or private equity firms are now eligibleto apply to the NIH SBIR program and compete for up to 25% of NIH’s SBIR set-aside.
back to top 

Healthcare providers are taking telemedicine to new heights, with the market seeing growth of a whopping 237 percent within a five-year period, according to a new Kalorama report.
Officials say the telemedicine patient monitoring market grew from $4.2 billion in 2007 to more than $10 billion in 2012. According to the report, the market itself is considered small- to moderate in size but makes up for it with its notable number of competitors and “increasing awareness of effectiveness.”
back to top 
Investing in biotechnology is a riskier bet these days. The community of life sciences venture capital firms is contracting, despite scientific advances across many fields like g enomics, immunology, and diagnostics. Many promising new enterprises fail to produce marketable drugs, and even successful therapies may struggle to gain markets in an environment of health care cost cutting. enomics, immunology, and diagnostics. Many promising new enterprises fail to produce marketable drugs, and even successful therapies may struggle to gain markets in an environment of health care cost cutting.
That’s exactly why Johnson & Johnson (NYSE: JNJ) chose to expand its programs that nurture very early stage biotechnology and device startups in the Bay area, J&J executives said as they opened the company’s California Innovation Center in Menlo Park, CA, this week.
back to top 
Pathologists and clinical laboratory professionals who regularly analyze images will be interested in the findings of a research study designed to assess how the phenomenon called “inattentional blindness” among radiologists could cause them to possibly miss things hiding in plain sight.
‘Inattentional Blindness’ Occurs Even Among Highly-trained Radiologists
In a recent study, psychological scientists from Harvard’s Brigham and Women’s Hospital found that 83% of radiologists didn’t notice an image of a gorilla embedded in a computed tomography (CT) lung scan.
back to top 

Health Tech Hatch, a site launched last fall as a crowdfunding site specifically for health startups, is joining forces with one of the biggest crowdfunding platforms on the web, Indiegogo.
From the beginning, the company planned to help health startups both crowdfund and beta test their products with patients and physicians. But now, founder and CEO Patricia Salber said Health Tech Hatch plans to focus more closely on the beta testing side, while working on the crowdfunding piece through Indiegogo.
back to top 

San Francisco digital health accelerator Rock Health is kicking off its fifth program next week, and it has some unique startups in the mix.
Bound to be the most talked about is Augmedix, which is developing a healthcare app for Google Glass. Co-founders Ian Shakil and Pelu Tran haven’t said much about exactly what kind of app they’re working on, but it apparently will leverage Glass’s augmented reality and voice activation to help doctors keep their focus on patients. Augmedix has already raised $55,000 from 32 Upstart backers.
back to top 

More than 60 companies showed off their health data applications at Datapalooza IV this week and Krishna Yeshwant, a partner at Google Ventures, was the MC for a demo session on Tuesday afternoon.
As one of the presenters was fighting with his PowerPoint demonstration and the projection system, Yeshwant answered a few questions about opportunities for healthcare startups with the search company’s investment group. He said that the outlook has changed for healthcare IT startups now that Obamacare is here to stay.
back to top 

Through collaboration between Johnson & Johnson Innovation and the California Institute for Quantitative Biosciences (QB3), Janssen will create a 5,000-square-foot lab space within QB3’s 24,000-square-foot incubator space in San Francisco.
The company said that the new Bay Area incubator will use the San Diego model for its innovation space.
back to top 

HHS Secretary Kathleen Sebelius opens Health Datapalooza IV with an emphatic introduction speech asking those in attendance to continue driving more ideas and innovation in data and information exchange to improve the future of the US health system.
back to top 
 ROCKVILLE, MARYLAND, June 4, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, is proud to announce the publication of the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide was developed by BHI, the Economic Alliance of Greater Baltimore and the Montgomery County Department of Economic Development, in collaboration with the Baltimore Business Journal. ROCKVILLE, MARYLAND, June 4, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, is proud to announce the publication of the Central Maryland BioHealth Entrepreneur’s Resource and Finance Guide 2013. The Guide was developed by BHI, the Economic Alliance of Greater Baltimore and the Montgomery County Department of Economic Development, in collaboration with the Baltimore Business Journal.
The Guide serves as a compendium of resources to biohealth innovators and entrepreneurs working to start and grow new companies and technologies in the region. This essential entrepreneurial resource includes a compilation of information on financial resources, university facilities and programs, economic development programs, and existing federal laboratory facilities and programs, as well as how to work with these assets. The Guide is one of the many projects BHI is developing to successfully transform the regional environment for biohealth startups through harnessing Central Maryland’s biohealth assets and establishing an entrepreneurial ecosystem.
The Guide has been distributed through the Baltimore and Washington business journal subscribers, and is being distributed to all regional partners. For your copy of the guide please download here or contact BioHealth Innovation for details on how to receive a hard copy.
back to top 
Wednesday, June 19, 2013, 06:00pm – 09:00pm
CoFoundersLab & Society of Physician Entrepreneurs proudly presents an educational and networking forum for entrepreneurs in healthcare and life sciences.
To make the most of your time, please browse CoFoundersLab.com and filter by “Meetup Groups – Society of Physician Entrepreneurs” before attending. If you have not created a profile on CoFoundersLab.com, please note that this is required in order for people to discover you before the event and seek you out during the networking portion of the event.
More Information
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 11
The Universities at Shady Grove Conference Center

June 11
QIAGEN’s Germantown Facility

June 12

June 13
Ritz-Carlton Hotel DC

June 13
Bethesda North Marriott
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
| | | | | | | | | |