|
|
|
Thursday, October 24, 2013, 08:00am – 04:00pm
The I2C Conference is a full-day event to help you get your technology moving forward. Come hear three in-depth panels on Innovation, Commercialization and Financing, including speakers from MedImmune Ventures, NASA/Goddard, NIH, Northrop Grumman Information Systems, University of Maryland and many more. Enjoy lunch with your Table Host to discuss issues affecting your innovation or small business with a subject matter expert from a federal lab, university tech transfer office, venture capital, business service organization and others. Tour the wealth of information in our Exhibit area. Apply to pitch your business for feedback at ConnecTech – spaces open for only 6 applicants until October 4th. Register today at the Early Bird rate of $30 to save $10 before the price increases on October 4th!
back to top 
 DreamIt Health Baltimore is a healthtech accelerator that will select up to ten startups from around the world to take up residence in the heart of Charm City and achieve in four months what might otherwise take years. The program is designed to help these teams tackle significant problems in the healthcare industry and achieve critical business milestones. We do this by enabling access to people and resources normally out of reach, by removing as many obstacles as possible, and with guidance from successful entrepreneurs who have been there before and done it before. Participants will have the opportunity to work closely with all corners of Johns Hopkins and tap into the region’s wealth of federal healthcare institutions including CMS, FDA and NIH. The capstone of the program, Demo Day, gives these teams the opportunity to unveil their products and progress before a few hundred early-stage investors and key industry figures. DreamIt Health Baltimore is a healthtech accelerator that will select up to ten startups from around the world to take up residence in the heart of Charm City and achieve in four months what might otherwise take years. The program is designed to help these teams tackle significant problems in the healthcare industry and achieve critical business milestones. We do this by enabling access to people and resources normally out of reach, by removing as many obstacles as possible, and with guidance from successful entrepreneurs who have been there before and done it before. Participants will have the opportunity to work closely with all corners of Johns Hopkins and tap into the region’s wealth of federal healthcare institutions including CMS, FDA and NIH. The capstone of the program, Demo Day, gives these teams the opportunity to unveil their products and progress before a few hundred early-stage investors and key industry figures.
For more information visit DreamIt Health Baltimore
back to top 
Christy  Wyskiel is the new senior adviser to the president for enterprise development at Johns Hopkins University. Wyskiel has previously worked as an investor in early stage and startup companies. She has also provided business and management support to startups, such as Hopkins spinout GrayBug. Wyskiel is the new senior adviser to the president for enterprise development at Johns Hopkins University. Wyskiel has previously worked as an investor in early stage and startup companies. She has also provided business and management support to startups, such as Hopkins spinout GrayBug.
Wyskiel officially takes on her new role leading Hopkins’ tech transfer office Jan. 1. But she’s already busy getting the lay of the land and helping map out what will come next for Hopkins with regard to technology commercialization.
back to top 

When Pascal Soriot took the reins of AstraZeneca last fall, he said he planned to corral firms with promising innovations in their pipeline to reinvigorate the Anglo-Swedish pharma’s offerings.
And so he has – to the tune of more than $1 billion.
Since the former chief operating officer of Roche AG was named CEO on Oct. 1, 2012, the firm has made five acquisitions, five collaborations with other pharmas to develop drugs, and entered two licensing deals. Six of the 12 moves are tied to cancer treatment developments, a core focus for the firm, while the remaining are in diabetes and renal, kidney, cardiovascular and respiratory diseases.
back to top 

Governor Martin O’Malley today joined with Montgomery County Executive Isiah Leggett and City of Gaithersburg Mayor Sidney Katz to announce that Emergent BioSolutions is expanding into a new headquarters building in Gaithersburg, and also plans to make improvements to its existing Research and Development site. As part of the expansion, the global pharmaceutical company will retain its existing 235 employees, and hire an additional 133 employees over the next five years.
“Emergent BioSolutions’ continued investment in Maryland helps solidify our position as a life sciences powerhouse,” said Governor O’Malley. “I am proud that Emergent BioSolutions is growing in Maryland, continuing to create jobs and working to develop life-saving vaccines that will give hope to millions of people around the world.”
back to top 

CareFirst BlueCross BlueShield will offer up to $1.5 million in grants to help medical providers develop telemedicine-based behavioral health services, the Baltimore insurer announced this week.
CareFirst wants to seed projects that use video conferencing or other technologies to allow doctors and others to diagnose and treat patients remotely.
back to top 

As an African-American man entering a Caucasian-dominated industry, Solomon Graham wasn’t intimidated when he founded Quality Biological in 1983, investing $10,000 of his own money to start the business.
The Gaithersburg company is one of the longest-operating biotechs in Montgomery County and has grown into a multimillion-dollar enterprise. It provides products and supplies for molecular and cell biology laboratories to use in infectious disease and cancer research.
back to top 

The latest of edition of Mtech News published by Maryland Technology Enterprise Institute.
back to top 

A prominent health system CEO implored the Washington region’s employers to not drop their company health plans in response to the Affordable Care Act, predicting disastrous consequences for the industry if they do.
So far, most companies aren’t taking that step. But enough have to raise the alarm for William “Bill” Robertson, CEO of Gaithersburg-based Adventist HealthCare, which operates two Montgomery County hospitals and network of affiliated services.
back to top 

“When I was a young entrepreneur, board meetings were by far the worst days of my life,” says Jeff Bonforte, the veteran company-builder who just sold his latest, Xobni, to Yahoo. “Board meetings are the height of insecurity for a CEO. Basically it’s a group of people who can both judge you and fire you based on that judgment.”
He’s had his fair share of bad experiences. At his first company, iDrive, he’d find himself every quarter standing in front of the room, sweating bullets, struggling to get through his meticulously-prepared slides. “It was a mess,” he says. “They’d just sit there and tell me how insufficient I was, how I needed to bring in someone more senior, or smarter. Then it just hit me. I don’t need this. I don’t need people to attack me for four straight hours. I need people who can help me.”
back to top 

MedImmune’s venture arm has jumped in to lead a $12.5 million round for a Chapel Hill, NC-based startup that is working on a new drug to treat a common ailment spurred by chemotherapy. G1 Therapeutics, which was initially seeded by Hatteras Venture Partners to the tune of $600,000, says that the new funds will finance its IND work and point the company to proof-of-concept data on a drug designed to protect against myelosuppression–the loss of blood cells–during chemo. Hatteras Venture Partners and Mountain Group Capital contributed to the round.
G1 was founded on the work of Norman Sharpless at the University of North Carolina in Chapel Hill and Kwok-Kin Wong at Harvard Medical School. They concluded that a cyclin-dependent kinase 4/6 inhibitor could play a big role in protecting the bone marrow of chemo patients.
back to top 

Under Armour Inc. is hunting for the techies that can help the company beef up its fitness tracking device known as Armour39.
Armour39, a digital performance monitor launched in March, tracks an individual’s heart rate, calories burned and workout intensity, and provides a “WILLpower” score that reflects how hard an individual trained during a workout.
back to top 

QIAGEN N.V. (frankfurt prime standard:QIA) today announced the Empowered Genome Community, which is a first-of-its-kind initiative to help people who have had their genomes sequenced share, explore, and interpret their data with researchers and each other. To highlight how the community can spark new biomedical insight, QIAGEN also released an open collaborative analysis of myopia in 111 people whose genomes were sequenced through Harvard’s Personal Genome Project (PGP), which is a public repository of well-phenotyped human genomes. Anyone – citizen scientist or full-time researcher alike – can directly review and help refine the analysis via QIAGEN’s Ingenuity® Variant Analysis(TM) (https://variants.ingenuity.com/community-myopia) with the goal of jointly publishing robust insights on myopia next year.
back to top 

Billionaire oilman T. Boone Pickens plans to give Johns Hopkins University $20 million to help spur potentially vision-saving research at the school’s Wilmer Eye Institute.
The gift will be included in the 85-year-old’s estate and will create an endowment to fund a T. Boone Pickens Scholars program supporting scientists.
back to top 

The Nobel Assembly at the Karolinska Institute announced today that Randy W. Schekman, a Howard Hughes Medical Institute (HHMI) investigator at the University of California, Berkeley, Thomas C. Südhof, an HHMI investigator at Stanford University, and James E. Rothman of Yale University are the recipients of the 2013 Nobel Prize in Physiology or Medicine for their discoveries of machinery regulating vesicle traffic, a major transport system in our cells.
According to the Nobel Assembly, this year’s Nobel Prize in Physiology or Medicine honors three scientists who have solved the mystery of how the cell organizes its transport system. Each cell is a factory that produces and exports molecules. For instance, insulin is manufactured and released into the blood and chemical signals called neurotransmitters are sent from one nerve cell to another. These molecules are transported around the cell in small packages called vesicles. The three Nobel Laureates have discovered the molecular principles that govern how this cargo is delivered to the right place at the right time in the cell.
back to top 

Osiris Therapeutics shares rose Friday morning after the company said it is selling some of its stem cell therapy technology, including its transplant treatment Prochymal, to Mesoblast Ltd. in a deal that could be worth more than $100 million.
Prochymal treats bone marrow transplant cells that attack the recipient’s body, and it is approved in Canada and New Zealand but isn’t being sold. Osiris said it wants to focus on businesses with the greatest commercial potential. Its remaining products include Grafix, which is used to treat chronic and acute wounds, Ovation, which is used in tissue repair, and Cartiform, a treatment for acute cartilage injury.
back to top 

There have been more than 30 initial public offerings of biotechnology companies so far this year, and there’s a line around the block of promising new entrants looking to debut on the public markets.
Angelika Warmuth/European Pressphoto Agency But don’t call it a bubble. Those in the know are calling it a boom, and saying the good times are likely to continue for biotech, even in the face of clinical setbacks and other bumps in the road.
back to top 

This Notice is to provide NIH’s extramural community with information on how NIH is resuming operations after the government shutdown.
eRA Systems Availability
eRA systems will be available for use by the applicant/grantee/reviewer community on Monday, October 21.
Rescheduling October Application Due Dates
All October grant application due dates have been rescheduled as follows: Standard Due Dates:
back to top 

Cleveland Clinic is in the midst of its annual Cleveland Clinic Medical Innovation Summit today at the new Global Center for Health Innovation. More than 1,100 entrepreneurs, investors, executives and clinicians have gathered for a show and tell of new ideas in the medical world. Cleveland Clinic Chief Information Officer C. Martin Harris talks about how the health system stays nimble on the innovation front.
Cleveland Clinic Innovations, the corporate venturing arm of Cleveland Clinic, was founded in 2000, and it has been hosting the Medical Innovation Summit since 2003. Since Cleveland Clinic Innovations was founded, it has:
back to top 

There is one certainty about the current regulatory process for drug approval in the United States and Europe: No one likes it.
Manufacturers are frustrated by the need for large, complex, and lengthy clinical-development programs that often hinge on meeting a single endpoint in one pivotal clinical trial. As a result, the cost to bring a drug to market has been estimated to be well over $1 billion — and it may be much higher. Patients and providers are disturbed by lack of timely access to medicines that show early promise in addressing significant unmet needs. Even regulators, who are responsible for enforcing the current structure, chafe at what manufacturers typically present to them: successful trial results in patients who are carefully selected to show the drug offers benefits but who are not very representative of the broader population likely to receive it. Payers then have a mess on their hands: pressure to pay for premium-priced medications that, when broadly employed, don’t offer much therapeutic benefit over existing alternatives.
back to top 

The research, by Gary Dushnitsky, Associate Professor of Strategy and Entrepreneurship, London Business School and his co-author Dr Alvarez-Garrido, is featured in the British Venture Capital Association’s report ‘The Missing Piece’ and finds that corporate venture capital is now the driving force behind cutting edge medical innovation.
Dr Dushnitsky, also Academic Director, Deloitte Institute for Innovation and Entrepreneurship, said, “Biotech start-ups are increasingly turning to corporate venture capital arms, which are steadily on the rise, while traditional venture capital funds are partially drying out. In a recent Nature Biotechnology study, my co-author and I find that the shift in funding patterns is resulting in an increase in scientific publications as well as patenting output.
back to top 

Nutty things are happening in biotech. Irrational exuberance has returned. Generalist investors with lots of money are suddenly buying these stocks first and asking questions later. Companies can fire off meaningless press releases, and be rewarded. I heard a big-time money manager talk the other day about a recent biotech IPO being one of the “best performers” in the market. It had a two-week track record, and had done nothing fundamental to earn its tag as a “best performer.”
If markets are driven by cycles of fear and greed, and I believe they are, we are in the greed cycle.
back to top 

Investors are pouring venture capital dollars into the Washington region at a pace not seen in more than a decade.
Companies across the Washington region have attracted $1.15 billion in the first three quarters of the year, more money than the first three quarters of any year since 2001.
back to top 

Investments in electronic health records, tools to help hospitals absorb physician practices anda group working with hospital systems to help them set up their own insurance plans. Those three health IT companies helped drive venture capital investment in the software sector, which hit a 12-year high in the third quarter. Medical device and biotechnology companies collectively marked their lowest investment for a nine month period since 2005, according to the MoneyTree Report by PricewaterhouseCoopers and the National Venture Capital Association. It is based on data from Thomson Reuters.
About $3.6 billion was invested in the software sector and there were 42o deals in the quarter.
back to top 

You can purchase 14 gallons of organic milk or 396 lollipops. You can give her 33 rides on the Ferris wheel at the state fair, or you can get him a couple of violin lessons. You could put the money in a savings account, you could buy her her very own LeapFrog LeapPad Explorer digital learning tablet, or you could buy enough pizzas to feed all of her friends on the block. So many options, so many choices.
I took that money and got my daughter’s genes tested, ordering up an analysis of the composition of her very small self and its odds of living a long and healthy life. And in so doing, I in some small way tied her fate to the success of the company doing the analysis, a genetic-testing startup called 23andMe in Mountain View, California.
back to top 

AstraZeneca and Taiwan’s National Research Program for Biopharmaceuticals (NRPB) today announced a collaborative program to support academic research proposals using open innovation as a catalyst for drug discovery. The program will connect expert physicians and scientists with a wide range of high-quality, small molecule compounds and biologics developed by AstraZeneca.
Successful research proposals submitted from academic institutes in Taiwan will be funded by the NRPB to explore new therapeutic uses for specific AstraZeneca compounds which may in turn lead to the development of novel therapies for patients. Further financial details were not disclosed. Areas of high interest include cardiovascular, metabolic, respiratory, inflammation, autoimmune, oncology, infection and neuroscience diseases.
back to top 

Each year, a committee of Cleveland Clinic doctors asks hundreds of their colleagues to weigh in on which emerging healthcare technologies they think will help shape their practice over the next 12 months.
Then, the committee evaluates nominations based on clinical impact, probability of commercial success, progress in commercialization and significant human interest, and produces a top 10 list announced at the end of the Clinic’s annual Medical Innovations Summit.
back to top 

Biocrossroads, which provides money and support to Indiana’s life sciences industry, announced the seven finalists who stand to gain startup funding in its annual New Venture Competition. Six have biotech or healthcare in their sights. On Oct. 21, five of these companies will compete for $25,000 and access to the Indiana Seed II Fund‘s staff and network for early-stage business support at the Indiana Life Sciences Summit. Second and third place in this competition will rake in $15,000 and $10,000 respectively.
“The New Venture Competition has proven to be a great way for us to find and reward promising companies, and is an excellent opportunity for the competingcompanies to gain some exposure within Indiana’s life sciences community,’ David Johnson, president and CEO of BioCrossroads, said in a release.
back to top 

StartUp Health, a New York-based incubator for health technology startups, admitted 14 new companies into its three-year development program this week. The program, which received 1,200 applications in the past year, aims to give new medical companies long-term mentoring and access to capital.
The new entrants include companies working on medication adherence, remote medical services and patient engagement in physical therapy. Cohero Health, a one-year-old company based in New York, aims to help kids with chronic asthma manage their condition.
back to top 

Your liver could be “eating” your brain, new research suggests.
People with extra abdominal fat are three times more likely than lean individuals to develop memory loss and dementia later in life, and now scientists say they may know why.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 23
Shady Grove Innovation Center

October 24
Universities at Shady Grove – Building II

November 13

November 18
Johns Hopkins University, Montgomery Campus
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

October 16, 2013 at 6:00 PM – 8:00 PM
Johns Hopkins – Montgomery County Campus
The National Capitol Area Local Chapter of SoPE in concert with the JHU Carey Business Schoo, MedChi, Zanvyl Krieger School of Arts and Sciences Center for Biotechnology Education, and the Medical Society of Northern Virginia present:
“The Health IT Explosion” What Big Providers Want: Value Proposition, Pricing & Closing the Deal!
Presenters:
Pete Celano, is an MBA from UVa, and has been in Digital Health for ten years. He was a co-founder of a start-up in Remote Patient Monitoring called BeClose.com (McLean, VA) and has been consulting primarily for big providers such as MedStar where he focuses on identifying solutions that Improve Outcomes, Reduce Costs, Enhance Revenue and Extend Access. He’s also consulted in Mobile Personal Emergency Response Systems, Home Testing for Obstructive Sleep Apnea, Holter Monitors, GERD drugs and Infusion Therapy.
Anand Iyer, PhD, currently serves as President and Chief Operating Officer of WellDoc Inc. WellDoc is a healthcare company that utilizes technology-based solutions to improve diabetes and other chronic disease outcomes and enhance a patient’s quality of life while systematically reducing healthcare costs. Prior to WellDoc, he was the leader of the global wireless solutions practice at PRTM Managment Consultants.
Joe Peterson, MD, is currently CEO of Specialists On Call (SOC), the nation’s premier provider of specialty physician consultations delivered via teleconferencing. Most recently a partner in United Westlabs, a hospital services company. Dr. Peterson has served on the Board of Directors of the Global Health Council, as a jurist for the Bill and Melinda Gates Foundation awards in public health, and on the Board of Directors of the Aids Action Coalition.
back to top 

The New York eHealth Collaborative (NYeC, pronounced “Nice”) is a not-for-profit organization, working to improve healthcare for all New Yorkers through health information technology (health IT).
Founded in 2006 by healthcare leaders, in partnership with the New York State Department of Health, NYeC receives funding from state and federal grants to serve as the focal point for health IT in the State of New York. NYeC works to develop policies and standards, to assist healthcare providers in making the shift to electronic health records, and to coordinate the creation of a network to connect healthcare providers statewide. The goal of NYeC is that no patient, wherever they may need treatment within the State of New York, is ever without fast, secure, accurate, and accessible information.
back to top 
 DreamIt Health Baltimore is a healthtech accelerator that will select up to ten startups from around the world to take up residence in the heart of Charm City and achieve in four months what might otherwise take years. The program is designed to help these teams tackle significant problems in the healthcare industry and achieve critical business milestones. We do this by enabling access to people and resources normally out of reach, by removing as many obstacles as possible, and with guidance from successful entrepreneurs who have been there before and done it before. Participants will have the opportunity to work closely with all corners of Johns Hopkins and tap into the region’s wealth of federal healthcare institutions including CMS, FDA and NIH. The capstone of the program, Demo Day, gives these teams the opportunity to unveil their products and progress before a few hundred early-stage investors and key industry figures. DreamIt Health Baltimore is a healthtech accelerator that will select up to ten startups from around the world to take up residence in the heart of Charm City and achieve in four months what might otherwise take years. The program is designed to help these teams tackle significant problems in the healthcare industry and achieve critical business milestones. We do this by enabling access to people and resources normally out of reach, by removing as many obstacles as possible, and with guidance from successful entrepreneurs who have been there before and done it before. Participants will have the opportunity to work closely with all corners of Johns Hopkins and tap into the region’s wealth of federal healthcare institutions including CMS, FDA and NIH. The capstone of the program, Demo Day, gives these teams the opportunity to unveil their products and progress before a few hundred early-stage investors and key industry figures.
For more information visit DreamIt Health Baltimore
back to top 
 Our Entrepreneur-in-Residence Ram Aiyar participated in a tech transfer series featured on BioCentury This Week discussing the need for accelerated innovations and the current lack of commercialization focus on commercially relevant technologies. The program was divided into 3 parts, Stuck in the Lab, Fresh Approaches, and Validation and also featured Dr. Alicia Loeffler and Rosemarie Truman. Our Entrepreneur-in-Residence Ram Aiyar participated in a tech transfer series featured on BioCentury This Week discussing the need for accelerated innovations and the current lack of commercialization focus on commercially relevant technologies. The program was divided into 3 parts, Stuck in the Lab, Fresh Approaches, and Validation and also featured Dr. Alicia Loeffler and Rosemarie Truman.
The three-part program can be watched by following the link below.
![BioCentury 10.13.13 - [1] Stuck in the Lab](http://www.biocenturytv.com/images/marquees/marq_101313_play.png)
Stuck in the Lab
back to top 

Are you a Maryland biotechnology company or research organization working to commercialize a technology/product?
Apply by October 17th for the 2013-2014 BioMaryland Biotechnology Development Awards.
The BioMaryland Center annually awards $50,000-200,000 through its Biotechnology Development program to a fund life sciences projects which advance the movement of research and development toward commercialization.
More than $5 million has been distributed to 28 organizations through the Biotechnology Development Awards program since its inception in 2010. The program has yielded multiple success stories—including a university spin-out and a local company securing $25M in funding while quadrupling the number of its employees.
back to top 

If Maryland is to meet the workforce demands of a growing cyber security industry, it’s going to have to offer students a hands-on experience in internships, one of the state’s top educators said on Tuesday.
“We need to start focusing on providing a significant amount of internships. It gives the students real-world experience,” said William “Brit” Kirwan said, chancellor of the University System of Maryland, to an audience at CyberMaryland 2013 in Baltimore.
back to top 

Johns Hopkins University ranks 15th globally out of 400 research universities in the recently released 2013-2014 World University Rankings compiled by London’s Times Higher Education. The position marks a one-spot improvement over JHU’s ranking in the weekly publication’s 2012-2013 list.
The top 15 features 11 American universities—including the California Institute of Technology, which claimed the top spot for the third year in a row—and four foreign universities—Oxford (third), Cambridge (seventh), and Imperial College London (10th) in the U.K.; and Swiss Federal Institute of Technology Zürich (14th) in Switzerland. Harvard, Stanford, and MIT joined Caltech and Oxford in the top five.
back to top 

Shares of MacroGenics are surging in Thursday midday trading after the biotechnology company raised $80 million in its initial public offering.
MacroGenics sold 5 million shares of stock for $16 per share. It had expected to sell 4 million shares for $14 to $16 each. The $80 million total does not include expenses or underwriting discounts.
back to top 
GlycoMimetics, Inc. announced today that it has filed a registration statement on Form S-1 with the U.S. Securities and Exchange Commission (SEC) for the proposed initial public offering of shares of its common stock. The number of shares to be offered and the price range for the proposed offering have not yet been determined. GlycoMimetics has applied to list its common stock on the NASDAQ Global Market under the ticker symbol “GLYC.”
Jefferies LLC and Barclays Capital Inc. are acting as joint book-running managers for the proposed offering. Stifel is acting as co-lead manager and Canaccord Genuity Inc. is acting as co-manager.
The offering will be made only by means of a prospectus. When available, copies of the preliminary prospectus relating to the offering may be obtained from Jefferies LLC, Equity Syndicate Prospectus Department, 520 Madison Avenue, 12th Floor, New York, NY 10022, by email at Prospectus_Department@Jefferies.com or by phone at 877-547-6340 or Barclays Capital Inc., c/o Broadridge Financial Solutions, 1155 Long Island Avenue, Edgewood, NY 11717, by email at Barclaysprospectus@broadridge.com or by phone at 888-603-5847.
back to top 

Bicoastal venture firm New Enterprise Associates owns 75.2 percent of GlycoMimetics Inc., making NEA by far the biggest beneficiary of the Gaithersburg biotech’s planned initial public offering.
The company revealed NEA’s outsized ownership in its IPO paperwork, filed on Friday. It is not yet clear whether the firm, which has a major office in Chevy Chase, plans to sell some of its 26.7 million shares in the offering.
back to top 

BioMarker Strategies announced the appointment of Jerry Parrott as President, Chief Executive Officer and Director, effective immediately.
According to a release, Parrott will report to the Company’s Board of Directors, which will continue to be led by Chairman Jack Davis, a co-founder and former Chairman and CEO of Dianon Systems.
back to top 

Noble Life Sciences (Gaithersburg, MD), in collaboration with ImQuest BioSciences (Frederick, MD), has successfully developed a neutropenic mouse thigh model of infection and demonstrated its use in a study evaluating vancomycin for the treatment of Staphylococcus aureus infection. The model will serve as a new Noble service for the evaluation of the efficacy of novel anti-microbial compounds in the treatment of microbial infections.
Complicated skin and soft tissue infections are frequently encountered in clinical practice and are a significant cause of morbidity and mortality in hospitalized patients. The neutropenic mouse thigh model of infection has been used extensively to test and benchmark antimicrobial drugs leading to a significant impact on our current knowledge of antimicrobial pharmacology. This model allows the quantitative comparison of different agents and different dosing regimes and the determination of the time-course of antimicrobial activity under conditions optimal for efficacy, i.e., neutropenia.
back to top 

If UMD researchers are well on their way to creating a robot that can wiggle through the brain to root out the tumors deep within, then anything is possible. Plankton crawled through Spongebob’s cranium and now Dr. J. Marc Simard, a neurosurgeon at the University of Maryland School of Medicine; Jaydev Desai, a roboticist at the University of Maryland; and Rao Gullapalli, a radiologist, believe they’re developing something that can do the same.
It was Dr. Simard who fist came up with the idea after watching a show on TV featuring plastic surgeons using sterile maggots to root out damaged tissue from a patient. “It sounds strange, but it’s a real thing,” he said in an interview with NPR. That’s when the lightbulb went off. “If I could train maggots to resect brain tumors I would. I can’t do that, so robotic maggots are the next best thing.”
back to top 

The University of Maryland (UM) BioPark announced today that the Catholic Health Initiatives (CHI) Center for Translational Research (CTR) has signed a lease to relocate from its current operational base at the University of Maryland St. Joseph Medical Center in Baltimore County to the BioPark in West Baltimore. The CTR, one of three entities within the CHI Institute for Research and Innovation (CIRI), collaborates with biomedical researchers to focus on the intersection of biomedical advances in omics-based diagnostics and precision medicine. Under the terms of the signed lease, half of the CTR’s overall anticipated 50-person work force will occupy more than 11,000 square feet of laboratory and office space within the BioPark.
“It’s exciting to add the CHI Center for Translational Research to our growing list of BioPark occupants,” said Jim Hughes, President, Research Park Corporation, University of Maryland, Baltimore. “This organization is national in scope – and yet we are able to offer them an ideal location that allows the Center to stay local – moving from Baltimore County to the UM BioPark. Here, they have access to the University of Maryland School of Medicine and Medical Center, as well as the opportunity to be part of a growing life sciences community.”
back to top 

Washington, D.C. has become one of the centers for high-tech innovation, spurring some of the biggest investments from venture capital firms in the country. In fact, according to The Atlantic Cities, D.C. ranks among the top 10 cities for venture capital funding.
With the influx of startups and entrepreneuers looking for funding, venture capitalists are beginning to leverage social media to brand their firm, position themselves as thought leaders, and attract the top talent in the city. With the help of Klout, an online-influence scoring site, we checked out which local VC firms are leveraging Twitter the best. Take a look at the factors involving Klout scoring here, and without furhter ado, here the highest ranking VC firms in D.C.
back to top 

British drugmaker GlaxoSmithKline will seek marketing approval for the world’s first malaria vaccine next year after trial data showed the shot significantly cut cases of the disease in African children.
The vaccine known as RTS,S was found, after 18 months of follow-up, to have almost halved the number of malaria cases in young children in the trial, and to have reduced by around a quarter the number of malaria cases in infants.
“Based on these data, GSK now intends to submit, in 2014, a regulatory application to the European Medicines Agency (EMA),” GSK, which has been developing the vaccine for three decades, said in a statement.
back to top 

October 29th, 6:00pm
National Center for Advancing Translational Sciences, Rockville, MD
Please join us for an enhanced networking reception with leaders from the Mid-Atlantic Region’s life science community. Take time to network with your peers and make new connections. After networking, Christopher Austin will give a short overview of the National Center for Advancing Translational Science (NCATS). He will speak more specifically on what NCATS will be doing, the goals and the impact of the life science community in terms of collaboration.
back to top 

Wondering where the venture capital funding has gone in the healthcare industry? According to CB Insights, it’s the following 10 cities–for Q3 ’13 at least. (Listed in order of amounts of deals; note that though San Francisco saw more deals, San Diego saw more funding.)
back to top 

I am sure many of you have watched an episode of Shark Tank on ABC. The show allows a startup entrepreneur to pitch their idea to a panel of five respected venture investors, who either like or don’t like the opportunity, and if they do, compete for the investment. Many of the times I have watched the show, I end up cringing watching these poor entrepreneurs become the victims of undermarket valuations or a rushed decision which makes for “good TV watching” for the viewers at home, but bad business decisions for the company. I wanted to compare Shark Tank to reality in the venture capital world, to confirm my assumption.
back to top 

A total of 87 applicants from more than 30 cities across Russia sought to land one of just three residency openings in the U.S.-Russia Innovation Corridor (USRIC), a collaborative innovation initiative led by American Councils for International Education. Of the 12 finalists selected for interviews, two startups and one university technology transfer office will take on a renewable three-month residency in USRIC.
Through USRIC, the residents will collaborate with U.S. partners and develop new markets, using the resources of the Maryland International Incubator (MI2) housed at the University of Maryland at College Park (UMD).
back to top 

DC Innovation Corps (I-Corps), the new, National Science Foundation-backed program aimed at translating the region’s vibrant research community into successful startups and licensed technologies, kicks off its first cohort this week at the George Washington University with 20 teams of inventors and current and aspiring entrepreneurs.
The cohort launches with a diverse mix of teams from the Children’s National Medical Center, Johns Hopkins University, University of Maryland, the George Washington University, Virginia Tech, George Mason University, and regional entrepreneurs from the Emerging Technology Center, Maryland Technology Enterprise Institute (Mtech) and bwtech@UMBC.
back to top 

The HIMSS Innovation Center opens today in Cleveland, a city, known around the world for the Cleveland Clinic and the Rock and Roll Hall of Fame, and one that prides itself on being a city of firsts.
HIMSS leaders who describe their 50,000-plus member organization of health IT professionals as “cause-based,” make no bones about their intent to shake things up in healthcare – more than a little bit.
back to top 

Kids today … are actually doing some amazing stuff. Take 15-year-old Jack Andraka, who recently won the grand prize of the Intel International Science and Engineering Fair for developing an early detection test for pancreatic cancer.
Andraka came up with the idea for the test after a close family friend died of pancreatic cancer. Using free online science papers, he formed a basis for the test, which looks for increased levels of a biomarker for pancreatic cancer in blood and urine. He contacted 197 scientists, seeking help with his research, and was rejected by each one, before Dr. Anirban Maitra at Johns Hopkins University agreed to donate lab space and help him develop his research.
(Photo Credit: Wikimedia Commons)
back to top 
Here’s what we know about the Affordable Care Act: 32 million Americans who would otherwise be uninsured will now have coverage. What you might not know is that Obamacare could also boost entrepreneurship by decoupling healthcare from employment.
How would that work? Existing research estimates that universal health insurance coverage could increase self-employment by as much as 3.5 percent. The reality is that many would-be risk-takers stay with their employers in large part due to the assurance of health insurance, in what economists refer to as “job lock,” or “entrepreneurship lock.” But, this pressure to be employed by a larger company is loosening as the Affordable Care Act makes it easier and less expensive to purchase individual coverage. Now, hopeful entrepreneurs can go out on their own in a far more efficient allocation of their skills, without gambling their own health coverage, or that of their family.
back to top 

Tuesday, November 5, 2013 from 8:00 AM to 11:00 AM (EST)
Join business, government and technology leaders for a high-level conversation on the transformation of health care through technology and innovation. Bloomberg Government’s first annual health care summit, “Mind the Gap: Connecting Health Care Policy with Next Century Innovation,” will convene health care innovators, medical professionals, and government officials who are helping to redesign U.S. health care during a time of innovation.
Panelists
- The Honorable Todd Park, Chief Technology Officer, The White House
- John Sculley, former CEO, Apple
- Elli Kaplan, CEO and Co-Founder, Neurotrack
- Dr. Keith Dunleavy, President, CEO and Chairman, Inovalon, Inc.
Join the conversation: #BGOVHealth
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 16

October 23
Shady Grove Innovation Center

October 24
Universities at Shady Grove – Building II

November 13
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
This workshop on Oct 9th. had been cancelled due to the Government Shutdown and will be rescheduled at a later date.
 With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards. With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards.
It’s useful to hear directly from NIH program officials on the current funding priorities per Institute. We have assembled program leads from NHLBI, NIAID, and NCI who can provide small businesses with more insight into their SBIR programs. They will also be available for one-on-one consultations at the conclusion of the event.
Event Details:
Wednesday, October 9th, 1:00pm – 4:00pm (EDT)
VisArts
155 Gibbs Street, 6th Floor
Rockville, MD 20850
(Overlooking Rockville Town Center)
back to top 

BioMarker Strategies announced the appointment of Jerry Parrott as President, Chief Executive Officer and Director, effective immediately.
According to a release, Parrott will report to the Company’s Board of Directors, which will continue to be led by Chairman Jack Davis, a co-founder and former Chairman and CEO of Dianon Systems.
back to top 
GlycoMimetics, Inc. announced today that it has filed a registration statement on Form S-1 with the U.S. Securities and Exchange Commission (SEC) for the proposed initial public offering of shares of its common stock. The number of shares to be offered and the price range for the proposed offering have not yet been determined. GlycoMimetics has applied to list its common stock on the NASDAQ Global Market under the ticker symbol “GLYC.”
Jefferies LLC and Barclays Capital Inc. are acting as joint book-running managers for the proposed offering. Stifel is acting as co-lead manager and Canaccord Genuity Inc. is acting as co-manager.
The offering will be made only by means of a prospectus. When available, copies of the preliminary prospectus relating to the offering may be obtained from Jefferies LLC, Equity Syndicate Prospectus Department, 520 Madison Avenue, 12th Floor, New York, NY 10022, by email at Prospectus_Department@Jefferies.com or by phone at 877-547-6340 or Barclays Capital Inc., c/o Broadridge Financial Solutions, 1155 Long Island Avenue, Edgewood, NY 11717, by email at Barclaysprospectus@broadridge.com or by phone at 888-603-5847.
back to top 

Fyodor, a Baltimore-based biotechnology company, announced today that the US National Science Foundation (NSF) has awarded the company a Small Business Innovation Research (SBIR) Phase 2 grant. The $729,000 funding will help accelerate Fyodor’s effort to develop and validate a noninvasive multi-disease urine-based diagnostic test for Acute Febrile Illness (AFI), enabling the differential clinical diagnosis of leading global health diseases like malaria, typhoid, dengue, and leptospirosis from a single urine specimen in patients with fever.
back to top 

Berlin, Germany, and U.S.A. – Epigenomics AG (Frankfurt Prime Standard: ECX, OTC: EPGNY), the German-American cancer molecular diagnostics company, announced today that it has entered into a joint commercialization agreement with Polymedco Inc., a leading provider of colorectal cancer tests in North America. Both companies will jointly commercialize Epi proColon®, Epigenomics’ blood-based test for colorectal cancer (CRC) screening, in North America.
back to top 

Chevy Chase-based New Enterprise Associates has joined in a $10 million financing for Cydan LLC, a Cambridge, Mass.-based orphan drug accelerator.
The additional funding comes months after the project’s launch in April, and brings its total financing to $26 million. Lundbeckfond Ventures and Bay City Capital led the most recent financing.
back to top 

As health systems and hospitals consolidate and battle for patients, they are starting to employ online consumer marketing efforts. A new Provider Web Presence Index is now available to track the success of providers.
Payer+Provider Syndicate has created the Provider Web Presence Index, which was released Tuesday, and evaluates the quality of the web presence of hospitals compared with competitors. The tool is based on a single indicator, which incorporates measures of web traffic, inbound linking and site relevance.
back to top 

These days, having a strategic investor or partner has become almost a matter of survival for healthcare startups.
Corporate investing and partnering has really taken off over the last few years as pharma and medical device companies have opened their doors and wallets to find innovation outside of their four walls.
back to top 

The University of Maryland School of Medicine’s Center for Vaccine Development announced on Thursday that it has received a renewed contract from the National Institute of Allergy and Infectious Diseases for its research and clinical studies.
“The University of Maryland’s Center for Vaccine Development has long been a partner of the federal government in the clinical evaluation of vaccines,” Karen L. Kotloff, a professor of pediatrics and medicine and the head of infectious disease and tropical pediatrics at the University of Maryland’s School of Medicine, said. “Renewal of our contract is a testimony to our expertise in helping protect people throughout the world against diseases that pose significant public health threats.”
back to top 

After nearly 17 years, Elaine Amir, executive director of Johns Hopkins University Montgomery County Campus, retired, effective Sept. 30. Elaine has been the face of Johns Hopkins in Montgomery County and a leader on several community boards and initiatives. Through her work, she touched many lives, both personally and professionally.
Before she left, Hopkins Happenings asked her to look back on her years leading JHU MCC:
back to top 

Donald E. Ingber, a professor at Harvard University, has combined advanced electronics and biology to create a “lung on a chip,” a breakthrough device that could safely allow precise tests of risky new medical treatments before they are tried out on humans.
Just as eye-opening as his work, however, may be his source of federal financing.
It’s not the National Institutes of Health, the $30-billion agency that is the largest provider of federal basic-research money to universities. Instead it’s the Defense Advanced Research Projects Agency, or Darpa, an agency one-tenth as large as NIH and responsible primarily for meeting the military’s technological needs.
back to top 

Cydan, LLC, an orphan drug accelerator that identifies and de-risks programs with therapeutic and commercial potential, today announced that the company has expanded its initial round of financing, bringing its total financing raised to $26 million. Cydan launched in April 2013 with a $16 million financing from New Enterprise Associates (NEA), Pfizer Venture Investments and Alexandria Venture Investments. New investors Lundbeckfond Ventures and Bay City Capital led the $10 million expansion of this round of financing and were joined by Cydan’s previous investors NEA and Alexandria Venture Investments. In conjunction with this investment, Lundbeckfond Ventures Managing Partner Mette Kirstine Agger, MBA and Bay City Capital Investment Partner and Managing Director Carl Goldfischer, M.D., are joining Cydan’s Board of Directors.
back to top 

Sequencing the human genome seemed like a discovery so important that it couldn’t be overhyped—we had, after all, transcribed the blueprint for human life—but biotech executives somehow managed the trick. William Haseltine, the founder of Human Genome Sciences, predicted in 2000 that he would halve the time and money required to bring a drug to market. Randy Scott of Incyte Genomics claimed that, “In 10 years, we will understand the molecular basis for most human diseases.”
Not quite. The cost of bringing a drug to market has increased dramatically, quibbles about accounting methods notwithstanding. The process still takes more than a decade. We already had a thorough understanding of diseases linked to single genetic sequences, such as Huntington’s and cystic fibrosis, but if anything, exploring the genome has taught us how complicated the relationship between genes and diseases really is. Last year, for example, researchers in Canada linked 71 genetic regions to inflammatory bowel disease, bringing the total to 163 and counting.
back to top 

An early-stage investment fund founded by Revolution has raised $200 million in commitments, blowing past its $150 million goal. The new fund, Revolution Ventures, will invest in technology firms, mostly under $10 million in revenue, that are seeking to disrupt traditional industries.
“We are doing the things that we’ve been doing for the last decade,” Revolution co-founder Steve Case said. “Trying to find early-stage companies that are using technology to disrupt traditional industries, create new business models and have all kinds of different aspects that we find interesting.”
back to top 

Two days into a government shutdown over resulting from the Congressional showdown over funding Obamacare, funding is at the front of mind for many researchers who rely at least partially on grants from National Institutes of Health and other government sources. A survey of 608 translational researchers revealed that although insufficient funding is a significant barrier to the path to commercializing their work, there are other related challenges that are just as critical to their future.
How can translational researchers improve the rate of reproducible results? What other factors are undercutting funding? Where can they find collaboration opportunities?
back to top 

The STEM workforce in the DC area is sizable and totals about 330,000 workers. Some of these employees are retiring, some are changing jobs, and others are getting promotions leaving positions open. The healthy STEM climate means that there are a lot of job opportunities at all levels. But where do your students find STEM jobs? On TCM’s targeted career center, CORE.
CORE is a comprehensive resource for finding all levels of biotechnology, technology, and business jobs in the Mid-Atlantic region.CORE provides detailed job and internship listings in 15 categories that are updated frequently and promoted through our social media channels for easy access. Many companies in the area that hire STEM workers are TCM members and are vested in hiring local talent. So prepare your students for success and direct them to CORE. In addition to job listings, we also offer timely career resource articles to help students meet the many challenges of their job search.
Want to show your students that you’re committed to their future? Purchase a banner ad on the CORE website to let local companies know about the courses, certificates, and degrees you offer to prepare your students for STEM jobs.
back to top 

Even people without diabetes could be at higher risk of dying from disease
High blood sugar has been linked to a greater risk of dying from several types of cancer, a potentially worrying finding for countries with growing rates of obesity.
Researchers from Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, USA and Yonsei University in Seoul, Korea made the discovery by following more than 1.2 million Koreans for 10 years.
back to top 

A veteran biotechnology reporter once complained privately that covering the industry was like watching grass grow—companies seemed to inch by slow degrees toward products and profits, sustained by a dwindling stream of funding.
For an antidote to that dreary picture, consider the swift developments at StartX Med, a life sciences accelerator program founded by Stanford University students in 2012. It’s a health care-oriented offshoot of StartX, the original student-initiated incubator program for researchers and others affiliated with Stanford.
back to top 

BIO’s announced agreement with National Science Foundation (NSF) to bring SBIR-funded, early-stage biotech companies to present at BIO Investor Forum 2013. Presenting companies in NSF track include ADMdx, Biodesy, Carmot, CertiChem, Jade Therapeutics, Nano3D Biosciences, Nanofiber Solutions, Parabon Nanolabs, Stemina, and Tymora Analytical Operations. All are funded by NSF and focused on drug discovery, diagnostics, and other platform technologies.
back to top 
When Sunnyvale, Calif.-based Intuitive Surgical hit the market in 1999 with its surgical robot, da Vinci, the company and many of its early adopters hailed the new technology as a revolution that would benefit patients, surgeons and the health care system as a whole. Da Vinci combines high-definition visual tools with robot-guided medical instruments that allow surgeons to do complicated procedures using a few tiny incisions. The da Vinci system, which is widely used in urologic surgeries such as the removal of prostate tumors, has been shown by Intuitive and outside researchers to reduce post-surgery complications and shorten hospital stays.
back to top 

The Department of Health and Human Services isn’t the only federal agency interested in funding medical research.
Defense Advanced Research Projects Agency, the Department of Defense’s primary innovation engine that’s responsible for developing new technologies for use by the military, also frequently undertakes project in biology, medicine and neuroscience.
back to top 

Improving health literacy and patient engagement and developing alternative ways to appraise apps and physicians are among the ideas that have reached the finals of a Robert Wood Johnson Foundation competition. The people and companies behind these ideas are vying for the opportunity to get funding as part of a program to fund transformative innovations in healthcare.
Among the judges are Angel investor Esther Dyson (@edyson), PatientsLikeMe Co-Founder and President Ben Heywood (@patientslikeme), Rhode Island School of Design President John Maeda(@johnmaeda), IDEO Life Sciences Chief Strategist Rodrigo Martinez(@rodrigoatcg), Games for Health Co-Founder Ben Sawyer (@bensawyer), Fast Company Staff Writer Ben Schiller(@btschiller) and NPR Science Correspondent Shankar Vedantam(@hiddenbrain).
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 11
Georgetown Conference Center

October 23
Shady Grove Innovation Center

October 24
Universities at Shady Grove – Building II
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

University of Maryland, Baltimore and MedImmune are pairing up for a five-year, $6 million collaboration on bioscience research.
The Gaithersburg-based drug company and the university will both put money and scientists toward joint research projects. The projects will focus on medical conditions and diseases in MedImmune’s wheelhouse, such as cardiovascular disease, respiratory problems and inflammation.
back to top 

The success of MenAfriVac shows vaccines can be developed outside Big Pharma’s walls, but, in most cases, the cost of late-phase trials is too great for charities. Recognizing this, JPMorgan Chase and the Bill & Melinda Gates Foundation have set up a Big Pharma-backed investment fund.
GlaxoSmithKline ($GSK), Merck ($MRK) and Pfizer’s ($PFE) foundation are among the investors in the fund, which will back late-stage development of technologies to fight disease in low-income countries. Having raised $94 million from its initial backers, the Global Health Investment Fund (GHIF) will now start trying to give vaccines and other technologies the financial clout to navigate Phase III trials.
back to top 

GL-2045 is Gliknik’s Lead Recombinant Stradomer™ Designed to Improve on Pooled Human Intravenous Immunoglobulin
Gliknik Inc., a privately held biopharmaceutical company, today announced that it has entered into an exclusive worldwide licensing agreement with Pfizer Inc. for GL-2045, Gliknik’s recombinant stradomer™, a drug candidate that is designed to replace and improve on pooled human intravenous immunoglobulin (IVIG). GL-2045 has shown promising results in a broad range of preclinical tests and is being developed as a potential treatment for a wide variety of autoimmune diseases, including those in which IVIG is clinically used.
“GL-2045 is the first of several innovative drug candidates Gliknik is advancing for people with autoimmune diseases and cancer,” said Gliknik CEO David S. Block. “We selected Pfizer as our partner to progress GL-2045 from among several interested and capable parties because of its exceptional development, manufacturing and commercial capabilities.”
back to top 

Emergent Biosolutions, the maker of the only FDA-licensed anthrax vaccine, is expanding its footprint in Montgomery County, with a little bit of financial help from the state and county. Emergent is taking 50,000-square feet (the precise number was reported by the Washington Business Journal earlier this month) at 400 Professional Dr., a 129,360-square foot, five-story building here, owned by Corporate Office Properties Trust. It will be moving 112 of its current employees to the building in early 2015. Emergent plans to purchase building, which is adjacent to its current research and development facility located at 300 Professional Dr.
What is interesting about the deal is the rare glimpse it reveals about the type of state and county incentives available for such transactions: the State of Maryland contributed $2 million, Montgomery County $750,000 and the City of Gaithersburg $250,000.
back to top 
At a time when many states are looking to their research universities as sources of innovation and entrepreneurship, it is great to see that the University of Maryland is finally being recognized nationally. Howard Marks, an experienced entrepreneur, created the 2013 StartEngine College Index and identified the University of Maryland College Park as the top public university. I might add that the top private university is Northwestern, a fellow member of the BIG 10, which UM is joining as of 2014.
-Brian Darmody, Associate Vice President for Corporate and Foundation Relations, University of Maryland

As the Managing Director at a tech accelerator, I face a dilemma. I need to fund the most talented people, because they’ll be most likely to build great companies. But I also need to find the hidden gems. I’m the sort of person who’s always looking for the best deals. Let me tell you, great deals almost always come from unexpected sources.
Here’s the problem: If I look for talent in the same places everyone else does — at Stanford, Harvard, and MIT — I’ll have to fight crowds of investors and even other accelerators who are trying to do the same thing I am. I don’t like lines, and I hate waiting, so I don’t go with the crowd. I try to create value where others don’t recognize it.
back to top 

Anyone can have a great business idea, and now students don’t have to be enrolled in the business school to get help making their ideas a success. This semester, the business school’s Dingman Center for Entrepreneurship expanded its Innovation Fridays program to reach students of all majors. The program, which used to be advertised only to business school students, gives student entrepreneurs free consultations with successful business owners to get advice for starting their own small businesses, promoting social causes or creating new technology.
back to top 

Earlier this year Sun Yat-sen University, a well-regarded institution in Guangzhou in the Guangdong province of China, announced that the university and affiliated hospitals were entering into a novel collaboration with Johns Hopkins Medicine. The agreement would see Hopkins faculty working bilaterally with Sun Yat-sen’s medical faculty both in China and at Hopkins in order to help the university become a world-class biomedical research institute. The deal has significant implications for U.S. hospitals because, facing declining revenues, international collaborations like these offer a new path for growth.
It was the 30th major, revenue-producing, international healthcare collaboration for Johns Hopkins Medicine, with several more currently under negotiation — when the rest of the world combined has perhaps a few dozen similar partnerships. One reason Hopkins is outpacing others is because it created an agile satellite unit – Johns Hopkins Medicine International (JHI) – within the much larger parent organization solely dedicated to these projects.
back to top 

Northrop Grumman Corporation (NYSE:NOC) and the University of Maryland, Baltimore County’s Research Park Corporation – also known as bwtech@UMBC – hosted a ceremony today for the first graduating class of the Cyber Cync Program: AccelerEyes, Five Directions and Oculis Labs.
The event also marked the expansion of bwtech@UMBC’s Cyber Incubator program, a sign of the program’s success and the positive economic impact both initiatives are making on the region.
back to top 

What we’ve got in our corner: Everything from good schools to a really funky music scene.
What we need to do: Everything from reform our corporate tax structure to rearrange our offices.
What’s at stake: The economic drivers who will keep Baltimore, and Maryland, competitive with the rest of the country and the rest of the world.
back to top 

Newt Fowler looked across Baltimore’s Renaissance Hotel ballroom and noted who was missing from the tables.
Sure, there are plenty of leaders in today’s innovation economy in Baltimore, Fowler told the crowd Thursday at the Greater Baltimore Committee’s Economic Outlook Conference.
“They’re just not in this room today,” he said.
back to top 

Baltimore’s Sage Growth Partners sees opportunity where others see a headache.
Over the past few years Sage, a health care technology consulting firm, has seen business pick up as more health care providers look for help installing and managing the new electronic record systems. CEO Don McDaniel declined to disclose the company’s revenue but said Sage has seen 50 percent annual growth for the past five years. That’s a trend he expects to continue as the company makes a move to break into consulting for startup companies in the health IT sector.
back to top 

As mobile health advocates clamor for scientific proof to support their emerging field, Johns Hopkins University has introduced mHealth Evidence, an online reference tool designed to help researchers quickly locate literature demonstrating the feasibility, usability and efficacy of mobile technologies in healthcare.
After a soft launch in June, the Center for Communication Programs at Johns Hopkins Bloomberg School of Public Health in Baltimore this week formally introduced mHealth Evidence via the school’s federally funded Knowledge for Health (K4Health) project. “We wanted to have one, designated site to bring together mHealth evidence,” Heidi Good Boncana, a program officer for strategic communication, ICT and innovation in the Center for Communication Programs, told MobiHealthNews.
back to top 

The National Institutes of Health has launched a major initiative to improve how basic science advances and discoveries are translated into commercially viable products that improve patient care and advance public health.
The NIH Centers for Accelerated Innovations (NCAIs), funded by the NIH’s National Heart, Lung, and Blood Institute (NHLBI), will target technologies to improve the diagnosis, treatment, management, and prevention of heart, lung, blood, and sleep disorders and diseases.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices (NOT):
Requests for Applications (RFAs):
Program Announcements (PA):
back to top 

mHealth advocates are giving good early reviews to the U.S. Food and Drug Administration’s final guidance document on the regulation of mobile medical apps, with one expert calling it “an expansive document that truly seeks to deregulate our nimble and innovative industry, while ensuring patient safety.”
“The guidance goes much further than I thought it would,” said Robert Jarrin, senior director of government affairs for Qualcomm, who noted that the agency has taken a new and novel approach in launching a consumer-facing website with an adjoining list of regulated apps that may be updated on a regular basis. In addition, he said, the FDA is creating a team that will be tasked with answering public inquiries about mobile medical apps submitted through mobilemedicalapps@fda.hhs.gov.
back to top 

October 1 and 2, 2013 8:00pm ET/5:00pm PT
Now on its eighth run, the NIH SBIR Phase I Program for First-Time Applicants is a very practical step-by-step, four-hour online “How-To” workshop over two evenings to help researchers, faculty members, graduate students, post-docs and entrepreneurs create a SBIR company and apply to the NIH SBIR program in December of 2013. This workshop includes a post-course review of the applicant’s proposed SBIR application by our experts before submission to the NIH. As an added benefit, your SBIR companies will be included on NCET2’s newsletters that is sent out to VCs, angel investors, Global 1000 companies, and government funders.
The NIH SBIR/STTR program is one of the federal government’s best mechanisms to continue funding innovative life science research after traditional research funding has been exhausted. The objective of the program is to dramatically increase the impact of innovations derived from original federally funded R&D, and as such is an ideal program to fund university commercialization of research through new university/faculty/student startup companies. Phase I can be for up to $150,000 for 6 months. Phase II can be for up to $1 million for 2 years. After Phase I and II, the company should have eliminated enough technical and scientific risk of the original research that the company is ready for outside investor funding or product sales in the company sustainability final Phase III of the SBIR program.
back to top 

Something as mundane and common as giving and receiving compliments may be a serious challenge if you are an immigrant.
Over the years, I have learned, often the hard way, the essential importance of receiving and giving compliments, at home, at work, and in social or semi-social environment. Generally speaking, East Asian cultures tend to be more reserved in expressing appreciations or affection toward others compared to the American culture (some generalization here). We have all heard of such jokes about Asian parents singling out the only “B” in the child’s report card amidst all other “A”s and demand to know why the child had failed to get straight “A”s, while American-born parents would emphasize on the progress or efforts rather than the outcome.
back to top 

Health IT groups and mobile application developers’ reaction to FDA’s release of final guidance for mobile health apps is mixed, the Baltimore Sun reports (Wells/Clarke, Baltimore Sun, 9/23).
Details of Final Rule
According to the final guidance issued Monday, FDA will focus oversight on apps that:
- Were developed to be used as accessories to regulated medical devices, such as apps that allow health care providers to make diagnoses by viewing medical images on smartphones or tablets; or
- Can transform mobile devices into regulated medical devices, such as apps that allow a smartphone to be used as an electrocardiography machine (iHealthBeat, 9/23).
back to top 

The United States Food and Drug Administration has finally released guidelines on how it plans to regulate thousands of new health-related smartphone applications.
After months of delaying its decision, the agency has determined that the vast majority of these health-related apps pose a negligible threat to consumers. Most of these “mobile medical” apps do not need federal regulation, the FDA found, so developers and investors can breathe a bit easier.
back to top 

How can you tell Maryland is becoming a hotbed for cyber security business?
Ellen J. Hemmerly said it’s obvious from the companies looking into University of Maryland, Baltimore County’s technology incubator.
“We’re attracting not only local and regional entrepreneurs,” Hemmerly said. “We’re getting more and more inquiries and tenants from out of state.”
back to top 
 These days, having a strategic investor or partner has become almost a matter of survival for healthcare startups. Corporate investing and partnering has really taken off over the last few years as pharma and medical device companies have opened their doors and wallets to find innovation outside of their four walls. These days, having a strategic investor or partner has become almost a matter of survival for healthcare startups. Corporate investing and partnering has really taken off over the last few years as pharma and medical device companies have opened their doors and wallets to find innovation outside of their four walls.
back to top 
 Bureaucracy is one of the dirty words in business. Nobody wants to publicly admit their company is bogged down with too many layers of management, or needs a dozen committees to sign off on every little decision. For a couple years now, I’ve been hearing entrepreneurs complain about suffocating bureaucracy in pharma. Bureaucracy is one of the dirty words in business. Nobody wants to publicly admit their company is bogged down with too many layers of management, or needs a dozen committees to sign off on every little decision. For a couple years now, I’ve been hearing entrepreneurs complain about suffocating bureaucracy in pharma.
back to top 
 A new investment fund structured by JPMorgan Chase & Co. (NYSE:JPM) and the Bill & Melinda Gates Foundation will, for the first time, allow individual and institutional investors the opportunity to finance late-stage global health technologies that have the potential to save millions of lives in low-income countries. A new investment fund structured by JPMorgan Chase & Co. (NYSE:JPM) and the Bill & Melinda Gates Foundation will, for the first time, allow individual and institutional investors the opportunity to finance late-stage global health technologies that have the potential to save millions of lives in low-income countries.
With $94 million committed by a pioneering group of investors – including anchor support from Grand Challenges Canada (funded by the Government of Canada), the German Ministry for Economic Cooperation and Development (acting through KfW) and the Children’s Investment Fund Foundation – the Global Health Investment Fund (“GHIF” or the “Fund”) will help advance the most promising interventions to fight challenges in low-income countries such as malaria, tuberculosis, HIV/AIDS and maternal and infant mortality. To help mitigate the risk of investing in the clinical development of new technologies, the Gates Foundation and the Swedish International Development Cooperation Agency have committed to partially offset potential losses in the Fund, which will seek a financial return for investors by targeting high-impact technologies with public health applications in both developed and emerging markets.
back to top 

President Obama signed the Jumpstart Our Business Startups (JOBS) Act into law on April 5, 2012. BIO advocated strongly for this new law, which includes several important policies designed to stimulate capital formation for growing businesses, including those in the biotech industry. Some of the new policies were self-effectuating, while others are awaiting rulemaking at the SEC. Below is a summary of the relevant provisions in the new law, along with a status update on the implementation process for each.
back to top 

Companies spent $294 billion on research and development performed in the United States during 2011, compared with $279 billion during 2010 (table 1). Funding from the companies’ own sources was $222 billion during 2010 and $239 billion during 2011; funding from other sources was $57 billion in 2010 and $55 billion in 2011 (table 2). Data for this InfoBrief are from the Business R&D and Innovation Survey (BRDIS), which was developed and cosponsored by the National Science Foundation and Census Bureau.
back to top 

The National Institutes of Health is making available approximately $3.7 million for awards to enhance training opportunities for graduate students and postdoctoral scholars to prepare them for careers in the biomedical research workforce that could take them outside of conventional academic research.
The first set of NIH Director’s Broadening Experience in Scientific Training (BEST) awards are supported through the NIH Common Fund’s Strengthening the Biomedical Research Workforce program.
back to top 

Venture capital for early-stage medical device companies is drying up. At Advamed 2013, I was able to sit down and talk to Paul Grand, managing director at Research Corporation Technologies Ventures, a life sciences firm focused primarily on medical devices. When I asked him what the three main mistakes startups make when pitching him, he sighed. His first response: “Only three?”
Ouch. So be sure to avoid these blunders when pitching VCs, startup CEOs:
back to top 

Covidien President and CEO Joe Almeida said in a little more than a decade, sub-Saharan Africa could be the big opportunity for medical device companies’ solution investments.
“It is a 10- or 15-year play. . . . The middle class will rise and you will have an opportunity,” Almeida said during the CEOs Unplugged series at Advamed 2013.
back to top 

At Rock Health, entrepreneurs are developing innovative products to keep us healthier, and lower medical care costs.
Ten health startups in Rock Health’s current accelerator class presented to a roomful of investors and the press today. Rock Health is a startup accelerator that focuses on health care technology.
The current class of startups are tackling huge challenges in health care, such as cancer treatment or eating disorders.
back to top 

Wednesday, October 30, 2013
8:30 AM to 11:30 AM
Do you love health technology? Do you want to learn more about it? Do you want to teach others and collaborate with local experts?
Call it what you want–mobile health, digital health, health IT–it’s all about using innovative technology to improve the lives of you, me, and the people we care about. Let’s build an ecosystem dedicated to making health technology part of everyday life and the standard of care! Being located in the Maryland area, we have all the pieces to the puzzle to promote innovation, collaboration, and investment in an industry that will revolutionize healthcare and impact the lives of all 7+ billion people around the world.
Join our ecosystem for the MD HealthTech Coalition Kickoff Event and hear from a panel of experts about the challenges, opportunities, and innovative solutions. More details to follow…
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 9
VisArts Rockville

October 11
Georgetown Conference Center

October 24
Universities at Shady Grove – Building II
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
Partnership with Johns Hopkins and BioHealth Innovation, Inc. will speed 10 healthtech startups to market

Ronald J. Daniels, President of Johns Hopkins
Baltimore, MD and Philadelphia, PA — September 18th, 2013—DreamIt Ventures is pleased to announce the launch of DreamIt Health Baltimore, a partnership with The Johns Hopkins University and BioHealth Innovation, Inc. to recruit, invest in, and speed the growth and success of a select group of early-stage health IT companies. The program comes on the heels of a successful health IT program in Philadelphia also built on strong industry partnerships that give participants access and advantages typically out-of-reach to startups.
“The key to making health care more accessible is innovation, and the most fertile focus for health care innovation is in acquiring, storing, analyzing and sharing information,” said Ronald J. Daniels, President of Johns Hopkins. “This accelerator project will have important implications for the future use of information as we use technology to find solutions for the most pressing health problems of our day. Just as important, it sets up Baltimore to become even more central to the health care information revolution through the rapid validation of solutions.”
“Technology holds the potential to transform the way in which we approach health care in this country and around the world,” said Paul Rothman, MD, Dean of the Johns Hopkins University School of Medicine and CEO of Johns Hopkins Medicine. “Johns Hopkins has been at the forefront in developing innovative solutions to the most pressing health care challenges. The partnership with DreamIt presents an exciting and unrivaled opportunity to develop the most cutting-edge solutions at the crossroads of information technology and medicine.”
back to top 
 Johns Hopkins University and BioHealth Innovation are partnering with Philadelphia-based DreamIt Ventures to bring a new health IT accelerator to Baltimore. The accelerator, called DreamIt Health Baltimore, is being supported by a portion of a $520,000 federal grant awarded to BioHealth Innovation and the Economic Alliance of Greater Baltimore. Johns Hopkins University and BioHealth Innovation are partnering with Philadelphia-based DreamIt Ventures to bring a new health IT accelerator to Baltimore. The accelerator, called DreamIt Health Baltimore, is being supported by a portion of a $520,000 federal grant awarded to BioHealth Innovation and the Economic Alliance of Greater Baltimore.
back to top 

A health IT accelerator is launching in Baltimore with the aim of pulling more technology out of Baltimore’s biggest research university and drawing more companies into the city. DreamIt Health Baltimore will host a class of 10 startup companies for a four-month accelerator program in Baltimore beginning in January. The accelerator is part of DreamIt Ventures outside Philadelphia.
back to top 

Aris Melissaratos is being replaced as Johns Hopkins University’s top technology commercialization adviser, as the university looks to delve deeper into entrepreneurship. Christy Wyskiel, an entrepreneur and investor, has been named senior adviser to the president for enterprise development at Hopkins. Beginning Jan. 1, Wyskiel will oversee Hopkins’ efforts to commercialize technology and research of faculty members.
back to top 

DreamIt Health, the healthcare-focused branch of Philadelphia startup accelerator DreamIt Ventures, is launching a Baltimore, Maryland class in partnership with Johns Hopkins University. BioHealth Innovation, an organization geared toward bringing more innovation and entrepreneurship to Maryland, is co-sponsoring the class with Hopkins.
back to top 
 With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards. With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards.
It’s useful to hear directly from NIH program officials on the current funding priorities per Institute. We have assembled program leads from NHLBI, NIAID, and NCI who can provide small businesses with more insight into their SBIR programs. They will also be available for one-on-one consultations at the conclusion of the event.
Event Details:
Wednesday, October 9th, 1:00pm – 4:00pm (EDT)
VisArts
155 Gibbs Street, 6th Floor
Rockville, MD 20850
(Overlooking Rockville Town Center)
back to top 

As a result of a strategic collaboration among the Maryland, Montgomery County and City of Gaithersburg economic development offices, Emergent Biosolutions – maker of the only FDA-licensed anthrax vaccine to protect against anthrax disease – will expand its headquarters in Montgomery County.
“Montgomery County was thrilled to partner with the State of Maryland and the City of Gaithersburg to provide Emergent Biosolutions with strategic funding to assist with their significant headquarters expansion in the County,” said Montgomery County Executive Isiah Leggett. “Emergent has the only FDA-licensed anthrax vaccine on the market, 235 current jobs, plans to add 133 new jobs over five years and was in the top 20 on the Washington Business Journal’s recent list of top 100 largest publicly traded companies; they are a poster-child for smart government investment, investment that will support both their continued contributions to global health and their continued contributions to the health of our local economy.”
back to top 

The University of Maryland has once again made The Princeton Review’s list of the country’s top entrepreneurship programs. In the 2014 list of the “Top 50 Schools For Entrepreneurship Programs,” published in Entrepreneur magazine, UMD ranks No. 15 for its undergraduate program. The university also ranks No. 16 for its graduate program, up eight spots from the 2013 rankings.
back to top 

The University System of Maryland played a role in launching or propelling about 180 startup companies in fiscal 2013, according to a new report from the university system.
The companies’ ties to a state university varied — some licensed technology developed at a university, others leased office space at a university research park and took advantage of the resources there, and still others were heavily coached and mentored by university experts.
back to top 

Sure, Johns Hopkins University is known for its medicinal prowess, but what better way to increase the university’s influence on the health care sector than by cosponsoring an accelerator aimed toward spurring the growth of more health information technology companies.
Johns Hopkins announced its plans to work with DreamIt Health Baltimore on Wednesday, a four-month long boot camp for innovators in the health IT business. Teaming up with BioHealth Innovation and DreamIT Ventures, Johns Hopkins will be powering the accelerator designed to fast-track promising ideas aimed at solving problems in America and abroad.
back to top 

The University of Maryland, Baltimore, has broken ground on its largest building ever, a $305.4 million, 10-story, 428,970-square-foot biomedical research facility called the Health Sciences Facility (HSF) III. University of Maryland President Jay A. Perman, MD, was joined by Gov. Martin O’Malley, Lt. Gov. Anthony Brown, University of Maryland School of Medicine Dean E. Albert Reece, MD, PhD, MBA, and several hundred invited guests at a groundbreaking ceremony Sept. 17 on the site of the new building ý the old dental school facility on North Pine Street.
“This is a proud day for the University of Maryland, Baltimore,” Dr. Perman told the crowd. The campus has expanded from 1.9 million square feet in 1975 to occupy 5.9 million square feet in 2013, he noted. “The University of Maryland, Baltimore, the University System of Maryland’s founding campus, has experienced robust growth in recent years. The Health Sciences Facility III further strengthens our footprint in west Baltimore and, as a result, our economic impact on the city and the state. We’re privileged to be able to help revitalize our critical important Baltimore neighborhoods and the state of Maryland as a whole, and at the same time, enable biomedical research and education that has the potential to save lives.”
back to top 

The University of Maryland (UMD) and Siemens Corporation announced today the largest ever in-kind software grant from Siemens PLM Software. The in-kind grant has a commercial value of more than $750 million. Siemens’ product lifecycle management (PLM) software will provide UMD students and researchers with a uniquely valuable and sophisticated design and simulation tool for course work, research, academic projects and team-based competitions.
This in-kind grant from Siemens gives students and faculty access to the same technology that companies around the world depend on every day to develop and manufacture innovative products in a wide variety of industries, including automotive, aerospace, biotechnology, machinery, shipbuilding, and high-tech electronics, among others.
back to top 

When GlaxoSmithKline made a clean sweep of Human Genome Sciences execs last year following its $3.6 billion buyout of the Rockville biotech, one big question (among many) was where would they land?
At least two have found their way back into Maryland biotechs. Last month, former HGS chief commercial officer Barry Labinger joined Anthrax-vaccine-maker Emergent BioSolutions as head of its bioscience division. And on Thursday, pre-IPO biotech MacroGenics announced the appointment of David Stump, formerly executive vice president for research and development at Human Genome, to its board of directors.
back to top 

To us, biomedical research is not an abstract idea funded by wasteful government spending. It is work done by driven and passionate young people like ourselves who want to save lives — but that work requires money, resources, and time.
We are a group of graduate students at the Johns Hopkins University School of Medicine’s Medical Scientist Training Program, earning our joint MD-PhD degrees with the goal of becoming physician scientists. We aim to be well versed in both scientific research methods and clinical practice so that we can expand and improve medical care, save lives, decrease the cost of health care and drive medicine forward. Thus far, the discussion surrounding the sequestration has lacked the perspective of trainees in biomedical research and the implications that budget cuts have had on our training and future careers.
back to top 

Rockville biotechnology company Emergent BioSolutions plans to buy a Gaithersburg building and move its 112 employees at its headquarters there, as well as add 133 jobs over the next five years, executives said Monday.
As part of the deal, the Gaithersburg City Council was expected to consider awarding a $250,000 economic development grant to Emergent during its meeting Monday evening.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
back to top 
 WASHINGTON – U.S. Senator Barbara A. Mikulski (D-Md.), Chairwoman of the Senate Appropriations Committee, today announced that the Economic Alliance of Greater Baltimore, Inc. and BioHealth Innovation, Inc. of Montgomery County have been awarded a combined $520,000 grant through the U.S. Department of Commerce’s (DOC) Economic Development Administration (EDA). The federal funding will be used to establish the Healthcare Regional Innovation Cluster (H-RIC) initiative which will combine the strengths of Maryland’s cutting-edge health research institutions and unique federal assets to speed the development of new treatments and cures, supporting Maryland jobs and keeping families healthy. WASHINGTON – U.S. Senator Barbara A. Mikulski (D-Md.), Chairwoman of the Senate Appropriations Committee, today announced that the Economic Alliance of Greater Baltimore, Inc. and BioHealth Innovation, Inc. of Montgomery County have been awarded a combined $520,000 grant through the U.S. Department of Commerce’s (DOC) Economic Development Administration (EDA). The federal funding will be used to establish the Healthcare Regional Innovation Cluster (H-RIC) initiative which will combine the strengths of Maryland’s cutting-edge health research institutions and unique federal assets to speed the development of new treatments and cures, supporting Maryland jobs and keeping families healthy.
back to top 

Arizona State University and Mayo Clinic announced today that Mayo will become a technology transfer participant to join Arizona Furnace, the startup accelerator that supports entrepreneurial teams using designated research discoveries and intellectual property as the basis for new companies.
As ASU and its current partners prepare to launch the second application season for AZ Furnace, Mayo Clinic will provide access to high potential technologies in their extensive intellectual property vault. These technologies, as well as those from ASU, Northern Arizona University and Dignity Health in Arizona, will be made available to entrepreneurs interested in using those discoveries to create products, services and new companies.
back to top 

Fenwick & West LLP, one of the nation’s premier law firms providing comprehensive legal services to high technology and life science clients, today announced the results of its First Half 2013 Life Science Venture Capital Survey.
The survey analyzes the valuations and terms of venture financings for 149 life science companies headquartered in the United States that reported raising capital during the first half of 2013, as well as trends in venture capital financings, fundraising and exit events.
back to top 

A report from the Agency for Healthcare Research and Quality (AHRQ), “Health IT-Enabled Quality Measurement: Perspectives, Pathways, and Practical Guidance,” outlines experts’ viewpoints on how information technologies are advancing the science of quality measurement. Over the course of the 2-year project, diverse perspectives were identified regarding how to operationalize quality measurement as well how to prioritize iterative advancements in health IT-enabled quality measurement.
Stakeholders agreed on the importance of addressing measure development, implementation, and testing; data elements and data capture; data access, sharing, aggregation, and integration; patient engagement; and collaboration and education. They also agreed that they would like to see quality measurement move beyond “checking the box” to truly support the quality improvement process. They suggested that quality measurement should be actionable and timely to allow patients and providers opportunities to improve care.
back to top 

The idea that technology will change medicine is as old as the electronic computer itself. Actually, even older. In 1945, Vannevar Bush, the man with the vision for the National Institutes of Health, foresaw a Memex computer program that would allow access to past books and records. A lone physician searching for a diagnosis in far-flung case histories was one of the applications Bush imagined.
Medicine is an information intensive industry. Yet there’s still no medical Memex. Even though the Internet teems with health information, study after study shows that medical care often differs greatly from what the guidelines say—when there are guidelines. Doctors frequently rely on their own experience, rather than the experience of millions of patients who have seen thousands of doctors. Not only is the past lost, the present is missing. How many times has a patient received a drug that causes an allergic reaction, just because that information is not available at the time it is needed?
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9-27

October 9
VisArts Rockville

October 11
Georgetown Conference Center

October 24
Universities at Shady Grove – Building II
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards. With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards.
It’s useful to hear directly from NIH program officials on the current funding priorities per Institute. We have assembled program leads from NHLBI, NIAID, and NCI who can provide small businesses with more insight into their SBIR programs. They will also be available for one-on-one consultations at the conclusion of the event.
Event Details:
Wednesday, October 9th, 1:00pm – 4:00pm (EDT)
VisArts
155 Gibbs Street, 6th Floor
Rockville, MD 20850
(Overlooking Rockville Town Center)
back to top 
 WASHINGTON – U.S. Senator Barbara A. Mikulski (D-Md.), Chairwoman of the Senate Appropriations Committee, today announced that the Economic Alliance of Greater Baltimore, Inc. and BioHealth Innovation, Inc. of Montgomery County have been awarded a combined $520,000 grant through the U.S. Department of Commerce’s (DOC) Economic Development Administration (EDA). The federal funding will be used to establish the Healthcare Regional Innovation Cluster (H-RIC) initiative which will combine the strengths of Maryland’s cutting-edge health research institutions and unique federal assets to speed the development of new treatments and cures, supporting Maryland jobs and keeping families healthy. WASHINGTON – U.S. Senator Barbara A. Mikulski (D-Md.), Chairwoman of the Senate Appropriations Committee, today announced that the Economic Alliance of Greater Baltimore, Inc. and BioHealth Innovation, Inc. of Montgomery County have been awarded a combined $520,000 grant through the U.S. Department of Commerce’s (DOC) Economic Development Administration (EDA). The federal funding will be used to establish the Healthcare Regional Innovation Cluster (H-RIC) initiative which will combine the strengths of Maryland’s cutting-edge health research institutions and unique federal assets to speed the development of new treatments and cures, supporting Maryland jobs and keeping families healthy.
back to top 

By Alissa Gulin, Daily Record Business Writer
A sizeable portion of a federal grant will go toward launching an intensive, 16-week accelerator program for health information technology entrepreneurs in Maryland.
Rich Bendis, president and CEO of BioHealth Innovation, said participants in the competitive program will come to Baltimore, meet with potential investors and partners and access the resources of Johns Hopkins Medicine, one of the partners in the accelerator. Officials will unveil more details of the program within a couple of weeks.
BioHealth Innovation, a Rockville-based group that acts as an intermediary between research institutions and the private sector, was one of two Maryland organizations that received a combined $520,000 grant, announced Wednesday, to help grow the state’s burgeoning biotechnology industry. The Economic Alliance of Greater Baltimore, an economic development group, was the other recipient.
back to top 

United Therapeutics Corporation announced today that the U.S. Food and Drug Administration (FDA) has acknowledged the resubmission of the new drug application (NDA) for treprostinil diolamine extended release tablets (oral treprostinil) for the treatment of pulmonary arterial hypertension. The FDA classified the resubmission as a complete, class 2 response to its March 22, 2013 complete response letter, and set a user fee goal date of February 16, 2014.
About United TherapeuticsUnited Therapeutics Corporation is a biotechnology company focused on the development and commercialization of unique products to address the unmet medical needs of patients with chronic and life-threatening conditions.
back to top 

The University of Maryland and Northrop Grumman will jointly announce the launch of the Advanced Cybersecurity Experience for Students (ACES), the nation’s first cybersecurity honors program for undergraduates, at a special event on Wednesday, September 25, 2013, from 10:30-11:30 a.m. in the Stamp Student Union Atrium on the University of Maryland College Park Campus.
The ACES program is designed to educate future leaders in the field of cybersecurity through rigorous, hands-on learning experiences, an intensive interdisciplinary curriculum, collaborative projects, and professional insight from industry and business leaders. The four-year honors program offers students a living-learning experience, giving them the opportunity to collaborate and work closely together as they pursue their advanced program of study in cybersecurity. The inaugural ACES cohort is comprised of 57 students. The ACES program is supported by a major grant from Northrop Grumman. For more information about the program, visit aces.umd.edu.
back to top 

The INNo program trains research scientists in the entrepreneurial skills needed to bring technology inventions and services to the healthcare market.
Participants in the INNo program learn to:
- Identify and evaluate the commercial potential of intellectual property
- Understand the business fundamentals related to technology start-ups
- Create a value proposition and business concept for a new product, platform, or service
- Articulate investment opportunities persuasively to potential investors and partners
- Develop a network of resources in the Maryland entrepreneurial community
back to top 

The Abell Foundation underwrote a comparison of Baltimore’s innovation ecosystem with that of Boston. Sean Pool and Matt Van Itallie, of Canterbury Road Partners, undertook the work and their report is telling of the challenges in front of us, but also reveals great promise of the resources we already have and what Baltimore’s innovation ecosystem can become. The full report can be found on the Abell Foundation website.
back to top 

This video is the first in a series about Startup Shell, the first student-founded, student-run venture incubator at the University of Maryland.
The Startup Shell is a vibrant co-working space at UMD where talented students guide their colleagues through ideation, research and development, product prototyping, business model creation and more.
Startup Shell is located in the Technology Advancement Program (TAP) incubator, an initiative of the Maryland Technology Enterprise Institute (Mtech) in the A. James Clark School of Engineering.
back to top 

University System of Maryland increased the number of new companies formed out of university technology by 29 percent in fiscal 2013 and is aiming to keep up the pace this year.
The university system credits the growth in new businesses in part to its heightened focus on entrepreneurship and transforming university research to commercial technology. Assistant Vice Chancellor for Information Technology Suresh Balakrishnan said the university system will look to continue adding new companies by looking for new ways to support startups, such as an investment fund.
back to top 

The Johns Hopkins University edged closer to the top 10 of national universities on the U.S. News and World Report annual rankings, which were released Tuesday.
U.S. News moved Hopkins up one spot to No. 12, just behind Dartmouth University and tied with Northwestern University, in the most-often cited of numerous college rankings. It was the highest ranking for Hopkins in 14 years. As expected based on prior rankings, Princeton, Harvard and Yale remained in the top three spots, in that order.
back to top 

Cars entering UMBC’s campus from I-195 will pass by a collection of nice looking buildings and a Subway restaurant. When temporarily stopped to obey the four-way stop before crossing the intersection, passengers may notice the sign that says bwtech@UMBC.
While the buildings and the sign are a familiar sight to many, their significance may remain unknown to those who pass by. These buildings house part of a research park run by UMBC named bwtech@UMBC.
back to top 

The National Cancer Institute (NCI) is interested in fostering communication between eligible small businesses and prime contractors.
The event, held in Rockville MD, will provide information about upcoming subcontractor opportunities for eligible small businesses, while assisting primes with meeting their subcontracting goals. NCI is interested in identifying a variety of small businesses that have expertise and capabilities in a range of specific NAICS codes listed.
back to top 

University of Maryland Received a 2013 NIH Grant for $219,084 for Age-Related Changes in Renal Morphology and Function in Chronic Kidney Disease. The principal investigator is Yu Chen. The program begain in 2012 and ends in 2014. Below is a summary of the proposed work.
This application is in response to PA-09-166 “Renal Function and Chronic Kidney Disease (CKD) in Aging (R21)”. CKD is a growing problem among the aging population. According to the United States Renal Data System, the number of older patients with end-stage renal disease (ESRD) has almost doubled over the last 25 years.
back to top 

Two Washington region tech companies — Clarabridge Inc. and Virtustream Inc. — rolled out a combined $120 million in funding on Tuesday, another sign of an improving venture ecosystem. Both rank among the Top 10 biggest D.C. Tech investments thus far in 2013
Not all of the fundings on this list qualify as “venture,” of course. Some of the larger ones could just as easily be called private equity, or at least the hybrid term “growth.” Like we see with Clarabridge’s $80 million, big-ticket financings like these often go partly toward cashing out earlier investors. And not all came from traditional VC. In Virtustream’s case, the entire $40 million Series D round came from a single corporate source: SAP AG.
back to top 

Maryland entrepreneurs are getting the chance to bring their small business plans directly to a panel of angel investors who could bring those ideas to life.
“The one thing we want to do is bring your story back with us,” said Mike Binko, founder and co-chair of Startup Maryland. “We want to put a big spotlight and a big megaphone in your hands to tell your story in your words.”
back to top 

In investing, comedy, and business, timing is a key component of success. It’s not enough to have a sense of where the future is going, directionally – you have to have some sense of when it’s likely to arrive.
In investing, this challenge is perhaps seen most vividly in bubbles, as Gregory Zuckerman details in The Greatest Trade Ever, providing example after example of exceptionally smart people — from Isaac Newton to Stanley Druckenmiller — who were able to correctly perceive a bubble, but who nevertheless lost huge sums of money by inaccurately estimating when it would burst.
back to top 

Virginia’s Center for Innovative Technology (CIT) has launched the first cyber-security technology startup accelerator program in the U.S. The Mach37 Cyber Accelerator is intended to locate and leverage the wealth of cyber security talent in Virginia to create companies that will develop and launch new products.
Mach 37 represents 37 times the speed of sound, which is approximately the same as the Earth’s escape velocity.
back to top 
I don’t think I’ve ever (in 20+ years of writing) reprinted a press release verbatim. Often I don’t even get to the end of them because of all the PR speak. However, today the Mayo Clinic sent out this list. It is concise, compelling and I can’t think of any way to improve it. The 10 ways the human genome map can affect diagnosis and treatment is an important reminder that we have come a long way in a short time, and that healthcare in the United States is not all about arguing about who gets care and who pays for it. don’t think I’ve ever (in 20+ years of writing) reprinted a press release verbatim. Often I don’t even get to the end of them because of all the PR speak. However, today the Mayo Clinic sent out this list. It is concise, compelling and I can’t think of any way to improve it. The 10 ways the human genome map can affect diagnosis and treatment is an important reminder that we have come a long way in a short time, and that healthcare in the United States is not all about arguing about who gets care and who pays for it.
In case you had any doubts that the $3.8 billion investment in the genome mapping project was worth it, consider these 10 advancements compiled by the Mayo Clinic.
back to top 
Normal adult cells have been reprogrammed to become stem cells inside live mice for the first time.
As stem cells can be coaxed into developing into almost any kind of cell, being able to prompt this behaviour in the body could one day be used to repair ailing organs including the heart, liver, spinal cord and pancreas.
“By doing it in situ, the cells are already there in the tissue, in the right position,” says Manuel Serrano at the Spanish National Cancer Research Centre in Madrid, and co-leader of the new work.
back to top 

Roche’s move to skip over Inovio’s (NYSE: INO) late stage drugs for cervical cancer and Hepatitis C and make a beeline for its preclinical DNA vaccines for prostate cancer and Hep B was initially a little puzzling. Investors have preferred to park their money further upstream when products are in the clinic and they’ve been derisked. Big pharma is always looking for ways to take costs out of research and development. But the prospect of utilizing new technology with the potential to improve treatment is appealing for drug developers that want to build up and strengthen their drug pipeline.
back to top 

Target has joined one of the growing trends in healthcare: innovation challenges. It announced on Monday two contests: one will seek a solution that helps people make positive lifestyle and prevention choices, while the other will gather ways to help people live well with a chronic condition.
The Target Simplicity Challenge will reward the creators of the winning ideas $25,000 apiece, their own Target-branded flip camera, and a chance to partner with Target to develop the concepts. The deadline for ideas is Oct. 24 and winners will be announced by January.
back to top 

Harvard University has partnered with several venture capital firms to launch The Experiment Fund. The Experiment Fund is starting with $10 million and was co-founded by Harvard alumni Patrick Chung (New Enterprise Associates) and Hugo Van Vuuren. Accel Partners and Polaris Ventures is also associated with The Experiment Fund.
Two of the world’s most prolific technology entrepreneurs, Bill Gates and Mark Zuckerberg, are known for being Harvard University dropouts. Why did they drop out? Possibly due to lack of resources at the University. Mark Zuckerberg moved to Silicon Valley from Harvard University to raise VC funding for Facebook in May 2004 and never looked back.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9-27

September 18-19
South San Francisco near the Airport

October 11
Georgetown Conference Center

October 24
Universities at Shady Grove – Building II
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards. With Fall approaching, everyone is preparing for their favorite things whether that’s football season, pumpkin carving, or more reasonable temperatures in Central Maryland. Fall is also a great time for the Small Business Innovation Research grant program throughout National Institutes of Health. In partnership with the NIH, BioHealth Innovation is sponsoring a fall gathering to talk about SBIR awards.
It’s useful to hear directly from NIH program officials on the current funding priorities per Institute. We have assembled program leads from NHLBI, NIAID, and NCI who can provide small businesses with more insight into their SBIR programs. They will also be available for one-on-one consultations at the conclusion of the event.
Event Details:
Wednesday, October 9th, 1:00pm – 4:00pm (EDT)
VisArts
155 Gibbs Street, 6th Floor
Rockville, MD 20850
(Overlooking Rockville Town Center)
back to top 

MEXICO-U.S. INNOVATIVE BUSINESS OPPORTUNITIES
Tuesday, September 17, 2013; 7:30 AM – 10:30 AM
Mexico is currently the 3rd largest goods trading partner to the U.S. with $494 billion in total trade (2012) and is the 2nd largest economy in Latin America. This Global Trade Forum will provide access to experts and information about the ways innovative businesses connect across borders. A distinguished presentation will be made by the Mexican Ambassador to the United States, His Excellency Eduardo Medina-Mora. Public and private sector representatives will focus on bio-tech and other industry sectors as panelists and conduct one-on-one sessions with attendees.
back to top 

September 5, 2013 marks Emergent BioSolutions’ 15th year of operations. Emergent BioSolutions is a global specialty pharmaceutical company seeking to protect and enhance life by offering specialized products to healthcare providers and governments to address medical needs and emerging health threats. Celebrate 15 years of innovation and growth with them by learning about their beginnings and their commitment to their mission – to protect life.
back to top 

BD Diagnostics, a part of Becton Dickinson and Company, announced on Tuesday that the U.S. Food and Drug Administration has cleared the launch of its BD ProbeTec Trichomonas vaginalis Qx Amplified DNA Assay.
The BD ProbeTec Trichomonas vaginalis Qx Amplified DNA Assay will be used in BD Diagnostic’s BD Viper System with XTR Technology to detect T. vaginalis DNA in endocervical, vaginal and urine samples. The BD Viper System is used by laboratories to test samples for T. vaginalis, C. trachomatis and N. gonorrhea and can also be used in batch mode to test for chlamydia and gonorrhea, trichomonas and herpes.
back to top 

Baltimore, Maryland-based mobile health company WellDoc announced a new board of directors this week to help the company further commercialize its recently launched BlueStar product. The new board members include two just retired PricewaterhouseCoopers executives and a Washington insider. Up until now WellDoc’s board has largely consisted of the company’s own management team, but the new board will continue to include the company’s founder and CEO Ryan Sysko and its president and COO Anand Iyer. The new board members, which officially joined over the course of the past month, are Dr. David Levy, Terry Lierman, Donald Almeida and Michael Greenebaum.
back to top 

Kicking off the fall semester, the University of Maryland is giving the thousands of innovative minds across campus new ways to share and explore their fearless ideas. Starting today, the university is expanding its popular “Innovation Fridays,” which give students the opportunity to meet with experienced innovators and entrepreneurs, get free and impartial advice, brainstorm strategies, and learn about available resources and funding.
“We are committed to cultivating a community of innovators on campus,” says Dean Chang, UMD’s associate vice president for innovation and entrepreneurship. “Similar to Google’s 20 percent time, which gave the world innovations like Gmail by encouraging employees to spend one day a week pursuing bold projects that were not necessarily related to their job description, we want to encourage students to take one day a week—Fridays—to pursue their own fearless ideas. The expansion of Innovation Fridays will allow more students across all disciplines to do just that.”
back to top 

Johns Hopkins University’s Whiting School of Engineering said Tuesday it will partner with Campbell & Co., a Baltimore-based investment firm, in a teaching and internship program.
Staff from Campbell will teach an investment science course at Hopkins during the fall semester and offer paid internships to as many as three Hopkins students during the year.
back to top 

Governor Martin O’Malley today via video kicked off the 2nd annual InvestMaryland Challenge, a national business competition that supports Maryland startups and strengthens a climate of innovation and entrepreneurship that has been ranked #1 in the country by the U.S. Chamber of Commerce for two years in a row. The Maryland Department of Business and Economic Development (DBED), through the Maryland Venture Fund and the BioMaryland Center, will award $400,000 in top prizes spread across four categories — Life Sciences, IT, General Industry and a new Cybersecurity category — as well as secondary prizes and awards from partners and sponsors. DBED is continuing its partnership with Inc. magazine to promote the Challenge to a national audience of entrepreneurs and small business owners. The application period opens immediately and will close December 6 and applications are available here.
back to top 

Startup Maryland (www.startupmaryland.org), an initiative driven By Entrepreneurs … For Entrepreneurs, today announced that UNDER ARMOUR® (NYSE: UA) has agreed to be the host venue for the 2013 Pitch Across Maryland™ Last Stop … Innovation Celebration.
The 2013 edition of the Pitch Across Maryland is the second annual state-wide tour and celebration of entrepreneurship and innovation communities.
“Referred to by FOX News affiliate WBOC-TV 16 as Opportunity on Wheels, the inaugural Pitch Across Maryland tour was an overwhelming success last year and this year’s offering is about to hit the road on September 9 with the first Stop on the Boardwalk in Ocean City, MD,” stated Julie Lenzer Kirk, Startup Maryland co-chair.
back to top 

Biotech venture capitalists, as a group, haven’t had much to cheer about the past few years. But this is shaping up to be the year the storyline changes, thanks to the ripple effect from the biotech IPO class of 2013.
The story of biotech venture capital over the past few years, as many readers know well, has been mostly about struggle. Many firms haven’t been able to scrape out returns, and they’ve reacted by grasping for new investment models, pushing partners out, veering into other sectors, or shutting their doors. The historic shift, which came about a decade after the genomics bubble, left only a few firms with the desire and capability to make the edgiest, riskiest, and potentially most innovative investments in life sciences.
back to top 
While U.S. job growth was less than economists expected in August – increasing by 169,000 – the healthcare sector remained a bright spot, adding 32,700 jobs, according to the Bureau of Labor Statistics’ jobs report issued Friday.
In the healthcare sector, ambulatory healthcare services had the most growth, with 26,600 added jobs. Hospitals saw the least growth, adding only 900 jobs in August. Home healthcare services and nursing and residential care facilities continued to be solid, adding 9,500 jobs and 5,200 respectively.
back to top 

The Center for Innovative Technology (CIT) announced today it will host an event with Virginia Governor Bob McDonnell launching the MACH37™ Cyber Accelerator on Thursday, September 12, 2013. The Accelerator is a program designed to facilitate the creation of the next generation of cybersecurity companies in Virginia. Supported by Governor McDonnell, the General Assembly appropriated $2.5 million to CIT to start and operate the Accelerator for two years, after which the Accelerator will secure private funding. MACH37 is the nation’s first public-private sector Cyber Accelerator program
back to top 

High Level Overview – Recapped
Challenge Phases
- Letter of Intent (LOI): Teams provide information regarding the members of their team and indicate how team members meet eligibility requirements. Teams also outline their intent to participate in the Challenge
- Business Plan (BP): Teams develop a 10-page business plan or business model canvas; a five minute “live” pitch; and a one minute elevator speech via recorded video
- Start-up (SU): Teams incorporate their start-up and apply for seed funding and the NIH start-up license or a license from the Clemson University Research Foundation (for this invention). In order to complete these three activities,the teams should refine their commercialization plan, development plan, regulatory strategy and comprehensive view of their management team and board of advisors
back to top 

There has been a sharp increase in venture capital funding of life sciences companies in the second quarter of 2013, according to a new report from PriceWaterhouseCoopers and the National Venture Capital Association.
San Diego companies were the third-most invested in, following San Francisco and Boston.
back to top 

Dr. Michael M. Gottesman has spent nearly four decades as a highly respected scientist at the National Institutes of Health (NIH), conducting important studies on how cancer cells resist destruction by several widely used chemotherapy drugs.
As chief of the laboratory of cell biology at the National Cancer Institute (NCI), Gottesman is the country’s premier researcher on the interaction of cancer and medications. He has developed molecular tools to define the drug-resistance genes found in individual cancers, information that is used to predict a patient’s response to therapy.
back to top 

We are currently enjoying a much needed resurgence in the biotechnology industry. The industry has been the best performing sector in the stock market for the last two years and it appears it will be again this year. This performance has enabled companies to raise capital, either publicly or privately, in record amounts. We expect the industry to raise more than $4 billion this year. The performance of the stock of companies that have executed initial public offerings is quite impressive. The average stock prices of the 23 life science IPOs through July are up over 50 percent from their offering price (on average) with only four trading below their offer price.
back to top 

Governor Dannel P. Malloy today signed new legislation creating a $200 million Bioscience Innovation Fund during a ceremonial event at the University of Connecticut Health Center (UCHC). The fund will make investments over the next 10 years in the form of grants, equity investments, loans and loan guarantees to foster innovation in smaller companies.
“Over the last two and a half years, we’ve taken great steps forward in reinventing our economy,” said Governor Malloy. “With the addition of Jackson Laboratories and the investments we are making in our flagship university, we are positioning Connecticut to be a leader in the creation of 21st Century jobs. The Bioscience Innovation Fund will allow us to build on the tremendous progress that’s being made across our state. Little by little, we are turning around years of stagnation and growing jobs for our residents.”
back to top 

NovoBioPharma, a global biopharmaceutical firm with locations in Cambridge, MA, Delaware, and Philadelphia, and UPstart at the University of Pennsylvania announced today that they entered into a synergistic collaboration in which NovoBioPharma provides critical guidance and strategic direction to select life science UPstart companies embarking on the commercialization of early stage opportunities. UPstart provides support for Penn inventors interested in pursuing entrepreneurial endeavors through ventures formed in partnership with Penn. NovoBioPharma will assist companies within UPstart to develop scientific and financially sound development plans to take these entities from proof of concept and beyond.
back to top 

The NFL, Under Armour and GE on Wednesday launched Head Health Challenge II, an open innovation challenge to award up to $10 million for new innovations and materials that can protect the brain from traumatic injury and for new tools for tracking head impacts in real time. The challenge is part of the Head Health Initiative, a collaboration to help speed diagnosis and improve treatment for mild traumatic brain injury.
Kevin Plank, Founder and CEO of Under Armour said: “As longstanding partners of the NFL and in collaboration with GE, we take great pride in our participation in the Head Health Challenge II. We are excited to harness the power of innovation and assemble the best minds in the world towards an effort to make the field of play safer across all sports and for all athletes.”
back to top 
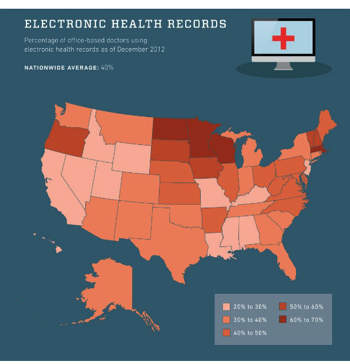
back to top 

This graph is a good visual depiction of how aging baby boomers, advances in technology and shifts in the way healthcare is delivered under reform may influence jobs in the healthcare sector.
In a Labor Day article, PBS directed us to interesting data published last year by U.S. Bureau of Labor Statistics in its Occupational Outlook Handbook (h/t @VentureValkyrie). Seventeen of the 30 occupations expected to grow the quickest between 2010 and 2020 are healthcare and medicine-related.
back to top 

Health care organizations in North America are projected to spend more than $34.5 billion on health IT in 2014 to comply with health care regulations, according to a new report from Technology Business Research, Clinical Innovation & Technology reports.
For the report — titled “SourceIT Healthcare Report” — researchers interviewed 225 health IT executives and line-of-business managers and conducted 25 in-depth interviews to learn about spending intentions, priorities and perceptions (Pedulli, Clinical Innovation & Technology, 8/29).
back to top 

In a financially stretched healthcare market, medical technology is sometimes seen as an expensive luxury. But use of the RIGHT technology can actually cut the overall cost of medical treatment and improve patient outcomes. You might be wondering how…
We live longer now, and we are more sedentary, so chronic diseases such as diabetes, chronic obstructive pulmonary disease, and Alzheimer’s are on the rise. These long-term degenerative diseases place a high cost burden on our healthcare systems. The sooner doctors can detect, treat, and/or prevent these conditions in patients, the more they can reduce this burden. This presents exciting opportunities for medtech companies to demonstrate R&D ingenuity.
back to top 

Sign up for the live Q&A Webcast >
Date & time: October 1, 2013 at 16:00 Central European Time (US: 10:00 EST, 7:00 PST)
In this live IM Channel One Ask the Expert Q&A hosted by NineSigma we will share hands-on experiences on how open Innovation can be applied to mitigate operator bias. By formulating business cases that address global healthcare needs, the pharmaceutical industry can open new avenues of innovation that are built on existing solid assets, developing accessory devices and services, creating user communities (medical and/or patient) and strategies to defend against the generic erosion of revenue.
back to top 

I visited cardiologist Eric Topol at the Scripps Green Hospital in La Jolla, California, one day this summer. He’d had a busy morning seeing patients and, by about noon, was claiming to have already saved the medical system tens of thousands of dollars using his iPhone and a pocket-sized ultrasound machine. Then he pointed to the stethoscope in his pocket and said he hasn’t used it in three years. “I should just throw it out,” he said. “This is basically a worthless icon of medicine.”
Topol is perhaps the most prominent advocate in the U.S. of how digital technology can lead to less expensive health care, and he invited me to see the savings in action. As we lope toward the exam room, Topol, slightly hunched and repeatedly turning to deal with questions flying at him from his staff, seems slightly rattled by the commotion and barrage of demands, but a calm sets in the moment he enters the exam room. He folds his arms across his chest as a young colleague updates him on the patient’s history. Topol introduces himself to the 85-year-old man, who has been tiring easily as of late, and then the doctor immediately pulls out his iPhone.
back to top 

Stanford University is going to start directly investing in students’ companies. Stanford is also giving a $3.6 million grant to StartX, a non-profit startup accelerator for Stanford-affiliated entrepreneurs.
StartX founder and CEO Cameron Teitelman tells me Stanford will only invest in StartX companies and alumni companies.
University spokesperson Lisa Lapin tells me Stanford’s investment fund will have an uncapped size. Vice President for Business Affairs Randy Livingston and his office will oversee the school’s investments, and Stanford will not take a lead role in funding rounds.
back to top 

Stanford University produces more alumni entrepreneurs than any other university, and now it’s created its first fund to invest directly in those early-stage companies emerging from the university community.
Stanford has established a three-year partnership with Stanford Hospital & Clinics and StartX, an accelerator for students, faculty, alumni and staff, under which the institutions will support the accelerator and create a fund called the Stanford-StartX Fund.
back to top 

The education establishment is in a twit over the phenomenon called “summer slide.” “Summer slide” is the loss of knowledge/competence in the summer months, according to research done by the RAND Corporation and Johns Hopkins University. “Summer slide” disproportionately plagues poor kids of color, so the recent, alarmingly low scores recorded by urban students on the Common Core tests heightened sensitivity around this issue.
Rather than acknowledging the corrosive effects of poverty, or conceding that the testing and accountability era has been a failure and has driven lousy practices, the answer is to make the least privileged kids work longer and harder while their more affluent peers summer in the Hamptons or South of France.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 11
William E. Hanna Jr. Innovation Center at Shady Grove

September 12
CIT

September 12
Novartis Institutes for Biomedical Research
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 Richard Moore, BD DiagnosticsROCKVILLE AND BALTIMORE, MARYLAND, August 26, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today its selection of Richard Moore, M.D., Ph.D., as a new Entrepreneur-in-Residence (EIR) at the National Institutes of Health (NIH) Office of Technology Transfer (OTT). BHI and BD established this position in July 2013. Dr. Moore, an executive with decades of experience in diagnostics development and technology strategy, will help support the development of new start-up companies and product commercialization based upon innovative technologies selected via OTT license agreements. Richard Moore, BD DiagnosticsROCKVILLE AND BALTIMORE, MARYLAND, August 26, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today its selection of Richard Moore, M.D., Ph.D., as a new Entrepreneur-in-Residence (EIR) at the National Institutes of Health (NIH) Office of Technology Transfer (OTT). BHI and BD established this position in July 2013. Dr. Moore, an executive with decades of experience in diagnostics development and technology strategy, will help support the development of new start-up companies and product commercialization based upon innovative technologies selected via OTT license agreements.
back to top 
AstraZeneca today announced that MedImmune, its global biologics research and development arm, has entered into a definitive agreement to acquire Amplimmune, a privately-held, Maryland, US-based biologics company focused on developing novel therapeutics in cancer immunology.
back to top 

Montgomery County Executive Isiah Leggett will visit China next month, leading a delegation of business and civic leaders looking for partnership opportunities in areas such as education and biotechnology.
While final details are still being worked out, Leggett’s group is scheduled to visit Beijing, Shanghai, Benxi and Xi’an, the county’s newest “sister city.” The trip, set for Sept. 15-25, has been jointly organized by the county, the Maryland China Business Council and the state of Maryland’s trade and investment office in Shanghai, which will help with roundtables and other contacts.
back to top 

ATCC, the premier global biological materials resource and standards organization, announces material deposit agreements with over 30 leading public and private institutions. These institutions will have the option to participate in the new Biomaterial Contributor Network (BCN), and make important research materials available to the research community. ATCC will coordinate with Technology Transfer Offices at each institution to create a simple, streamlined process for adding new microbial strains and cell lines to the ATCC collection. Many of the participants will have an opportunity to receive a share of the revenue from the sale and licensing of materials developed at their institutions. Over 225 unique biological materials are deposited under these agreements to date, with most available to both contributors and others as determined jointly by ATCC and the institution.
“Since 1925, ATCC has set the standard for providing the largest and most diverse collection of authenticated biological materials to the scientific community. The Network enables Contributors to create a lasting impact on science around the globe,” said Dr. Raymond Cypess, CEO of ATCC. “These agreements reinforce ATCC’s Mission to distribute scientifically valuable, authenticated materials, while recognizing the shared financial benefit with participating institutions,” said Matt Klusas, Senior Director of Corporate Development at ATCC.
back to top 
 Ten of Maryland’s nineteen fastest-growing companies are located in Montgomery County – including the state’s highest-ranking company and two of our incubator companies! Ten is also two more than made the list from well-publicized, regional rival Fairfax County, Virginia – supporting what local businesses and economic development professionals have known for years: Montgomery County is a GREAT place to grow a business. Ten of Maryland’s nineteen fastest-growing companies are located in Montgomery County – including the state’s highest-ranking company and two of our incubator companies! Ten is also two more than made the list from well-publicized, regional rival Fairfax County, Virginia – supporting what local businesses and economic development professionals have known for years: Montgomery County is a GREAT place to grow a business.
back to top 

Howard County has plans for a new incubator that would aim to develop companies with a socially conscious focus.
The Conscious Venture Lab is a partnership between Howard County and New York-based The Porter Group LLC. The incubator, which will be housed in the county’s Maryland Center for Entrepreneurship, could begin enrolling entrepreneurs and budding startups as early as this fall.
back to top 

AstraZeneca Plc took a further step to bolster its pipeline of new cancer drugs on Monday by agreeing to acquire privately held U.S. biotech company Amplimmune for up to $500 million.
The deal is the second within 24 hours in the cancer drug space, following Amgen Inc’s much larger acquisition of Onyx Pharmaceuticals Inc for about $10.4 billion.
Amplimmune specialises in developing treatments designed to help the immune system fight cancer and the purchase will give AstraZeneca access to a number of compounds currently in pre-clinical development.
back to top 
 BioMaryland is supporting various events in France which benefit the biohealth community. See if your company qualifies for a travel assistance grant to fly out and attend! BioMaryland is supporting various events in France which benefit the biohealth community. See if your company qualifies for a travel assistance grant to fly out and attend!
back to top 

A team of researchers led by Andrew Coop, PhD, professor and chair of the Department of Pharmaceutical Sciences (PSC) at the University of Maryland School of Pharmacy (UMSOP), has developed a new opioid drug that shows great potential to advance treatment and improve quality of life for individuals living with chronic pain. Spotlighted in a recent issue of ACS Chemical Neuroscience, the compound, known as UMB 425, is as strong as morphine, but displays diminished tolerance over time with no obvious toxic effects.
“UMB 425 is a breakthrough in the development of therapeutics to treat chronic pain,” says Coop (on the left in the photo). “Unlike other drugs developed to act on only one biological target, UMB 425 acts on two different opioid receptors in the body. When activated at the same time, these receptors work together to provide pain relief and slow the body’s development of tolerance to the drug. This diminished tolerance allows a lower dose of the opioid to be administered for a longer time period, while still achieving the same level of pain relief.”
back to top 

BD Diagnostics, a segment of BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, announced today the U.S. Food and Drug Administration clearance and launch of the BD ProbeTec™ Trichomonas vaginalis Qx Amplified DNA Assay for the direct qualitative detection of T. vaginalis DNA in endocervical and vaginal samples as well as neat urine specimens to aid in the diagnosis of trichomoniasis on the BD Viper™ System with XTR™ Technology. This assay has been CE-marked to the In Vitro Diagnostic Directive (98/79/EC).
Trichomoniasis is the most common curable sexually transmitted infection (STI). Worldwide, more than 180 million cases are estimated to occur annually.[i] Genital inflammation caused by trichomoniasis can increase a woman’s susceptibility to HIV infection. In HIV-infected women, trichomoniasis may increase the likelihood of HIV transmission to sex partners. Furthermore, trichomoniasis is often asymptomatic. For these reasons, experts recommend screening for T. vaginalis in women considered to be at high risk for infection (i.e., women who have new or multiple partners, have a history of STIs, exchange sex for payment, or use injection drugs).
back to top 

One of the first tenants to lease at Emerging Technology Center’s new Highlandtown location is SameGrain, developer of a social discovery platform that helps people anonymously locate, connect with, and grow new friendships with people who share similar demographics, beliefs, and interests.
One of SameGrain’s goals is making it easier for people who share common interests to find each other, says SameGrain co-founder Anne A. Balduzzi.
back to top 

Johns Hopkins researchers say they have found a specific protein in nearly 100 percent of high-grade meningiomas — the most common form of brain tumor — suggesting a new target for therapies for a cancer that does not respond to current chemotherapy.
Importantly, the investigators say, the protein — NY-ESO-1 — is already at the center of a clinical trial underway at the National Cancer Institute. That trial is designed to activate the immune systems of patients with other types of tumors that express the protein, training the body to attack the cancer and eradicate it.
back to top 

Forum Agenda: 7:30 a.m. – 5 p.m.
Jones Day Terrace & Conference Center
51 Louisiana Ave, NW | Washington, D.C.
With the increasing adoption of cloud computing, mobile devices and web-based applications, hackers have more opportunities than ever to infiltrate and crash network systems, especially in healthcare, which is increasingly becoming more vulnerable. The two greatest areas of opportunity for investment capital and the start-up community is in healthcare and cyber security. The nexus of these two sectors provides an even greater and more focused set of opportunities for investment. The Angel Venture Forum brings together all star roundtables of experts to opine and discuss the topics and the opportunities herein.
back to top 

NIH announces the availability of a Niche Assessment Program for its SBIR/STTR Phase I awardees funded in fiscal years (FY) 2013 and 2014. All active NIH SBIR/STTR Phase I awardees (by grant or contract) as well as those small businesses selected to receive a Phase I award in the first three months of the upcoming fiscal year will be eligible to participate. This program can help “jump-start” a company’s commercialization efforts by providing the market insight and data that can be used to strategically position its technology in the marketplace, by assisting companies with their development of commercialization plans for Phase II applications, and by introducing small businesses to potential partners.
A third party, unbiased assessment of appropriate market niches for products/services that are being developed by NIH’s SBIR/STTR Phase I awardees will be performed by Foresight Science & Technology. Using its Technology Niche Analysis® (TNA®), Foresight will perform the due diligence on markets appropriate for each SBIR technology and develop an in-depth report for each SBIR/STTR awardee that addresses:
- needs and concerns of end-users
- competing technologies and competing products
- competitive advantage of the SBIR/STTR-developed technology
- market size and potential market share (may include national and/or global markets)
- barriers to market entry (may include, but is not limited to pricing, competition, government
- regulations, manufacturing challenges, capital requirements, etc.)
- market drivers
- status of market and industry trends
- potential customers, licensees, investors, or other commercialization partners
- price customers are likely to pay
back to top 

Electronic medical records. DNA sequencing. Big data. These technology trends are changing the way medicine is practiced today — but what’s coming next? I scoured the web, reached out to futurists and drew from past conversations with industry leaders to compile a list of the next generation of disruptive technologies that are on the brink of breaking through in healthcare. What’s missing from this list?
back to top 

Novartis International AG / Novartis holds annual healthcare entrepreneur competition to generate insight into healthcare and innovation of tomorrow . Processed and transmitted by Thomson Reuters ONE. The issuer is solely responsible for the content of this announcement.
- The International Biotechnology Leadership Camp (BioCamp) fosters idea exchange with leading scientists as well as entrepreneurship for young talents
- 60 selected students from leading international universities attend to explore science and innovation at Novartis headquarters in Basel, Switzerland
- Novartis CEO presents innovative health care solutions and business approaches to address the evolving need to improve patient outcomes
back to top 
A new system for visualizing the brain during surgery is helping neurosurgeons more accurately diagnose and treat patients and is even allowing them to perform some procedures that until now have been extremely difficult or even impossible.
Neurosurgeons can use the imaging technology during surgeries that require small objects—biopsy needles, implants, or tubes to deliver drugs—to be placed at precise locations in the brain. The system provides live magnetic resonance images (MRI) that allow surgeons to monitor their progress during the operation.
back to top 
 This summer marked the inauguration of the DreamIt Health accelerator, a startup boot camp focused on healthcare IT run by DreamIt Ventures and powered by Penn Medicine and Independence Blue Cross. In four short months, ten extraordinary teams of entrepreneurs, including four from Wharton, were brought together from around the country to achieve significant milestones going from concepts to prototypes, products, pilots and revenues. As the program wound down, the investor, startup and healthcare community turned out in force for Demo Day to see a snapshot of each company’s progress and plans for the future. This summer marked the inauguration of the DreamIt Health accelerator, a startup boot camp focused on healthcare IT run by DreamIt Ventures and powered by Penn Medicine and Independence Blue Cross. In four short months, ten extraordinary teams of entrepreneurs, including four from Wharton, were brought together from around the country to achieve significant milestones going from concepts to prototypes, products, pilots and revenues. As the program wound down, the investor, startup and healthcare community turned out in force for Demo Day to see a snapshot of each company’s progress and plans for the future.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 11
William E. Hanna Jr. Innovation Center at Shady Grove

September 12
Novartis Institutes for Biomedical Research

September 18-19
South San Francisco near the Airport
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 Richard Moore, BD DiagnosticsROCKVILLE AND BALTIMORE, MARYLAND, August 26, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today its selection of Richard Moore, M.D., Ph.D., as a new Entrepreneur-in-Residence (EIR) at the National Institutes of Health (NIH) Office of Technology Transfer (OTT). BHI and BD established this position in July 2013. Dr. Moore, an executive with decades of experience in diagnostics development and technology strategy, will help support the development of new start-up companies and product commercialization based upon innovative technologies selected via OTT license agreements. Richard Moore, BD DiagnosticsROCKVILLE AND BALTIMORE, MARYLAND, August 26, 2013 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today its selection of Richard Moore, M.D., Ph.D., as a new Entrepreneur-in-Residence (EIR) at the National Institutes of Health (NIH) Office of Technology Transfer (OTT). BHI and BD established this position in July 2013. Dr. Moore, an executive with decades of experience in diagnostics development and technology strategy, will help support the development of new start-up companies and product commercialization based upon innovative technologies selected via OTT license agreements.
back to top 
AstraZeneca today announced that MedImmune, its global biologics research and development arm, has entered into a definitive agreement to acquire Amplimmune, a privately-held, Maryland, US-based biologics company focused on developing novel therapeutics in cancer immunology.
back to top 
 On Wednesday August 21st, 80+ people filled the BHI parking lot for an evening of networking, eating crabs, and enjoying a relaxed, stress-free atmosphere. Represented was a who’s who of the Montgomery County biohealth community and the local county government. On Wednesday August 21st, 80+ people filled the BHI parking lot for an evening of networking, eating crabs, and enjoying a relaxed, stress-free atmosphere. Represented was a who’s who of the Montgomery County biohealth community and the local county government.
Thank you to everyone who made it out and we hope to see you again next time!
back to top 
 This summer marked the inauguration of the DreamIt Health accelerator, a startup boot camp focused on healthcare IT run by DreamIt Ventures and powered by Penn Medicine and Independence Blue Cross. In four short months, ten extraordinary teams of entrepreneurs, including four from Wharton, were brought together from around the country to achieve significant milestones going from concepts to prototypes, products, pilots and revenues. As the program wound down, the investor, startup and healthcare community turned out in force for Demo Day to see a snapshot of each company’s progress and plans for the future. This summer marked the inauguration of the DreamIt Health accelerator, a startup boot camp focused on healthcare IT run by DreamIt Ventures and powered by Penn Medicine and Independence Blue Cross. In four short months, ten extraordinary teams of entrepreneurs, including four from Wharton, were brought together from around the country to achieve significant milestones going from concepts to prototypes, products, pilots and revenues. As the program wound down, the investor, startup and healthcare community turned out in force for Demo Day to see a snapshot of each company’s progress and plans for the future.
back to top 

Renee M. Winsky, a Maryland business executive with broad experience leading organizations, was named Leadership Maryland’s president and CEO.
She will replace Nancy Minieri, who founded the organization in 1992 and announced in March that she will retire at the end of this year.
back to top 
You sit down to a family dinner with aunts, your grandmother, three second cousins once removed, a floating niece, maybe a family dog. You’ve got great news: You’ve been accepted to Johns Hopkins University, a school that people who don’t live in the mid-Atlantic region know about, a school foreigners desperately want to get into. It’s ranked 13th in the world by the most recent U.S. News and World Report, for God’s sake; it’s definitely time to celebrate.
Your whole family remembers hearing Hopkins’ name in that Prince and Me movie with Julia Stiles, where she wanted to be a doctor instead of just marrying a prince and being rich and stuff. I guess that’s kind of what Hopkins is about, the whole getting educated and doing things instead of marrying a rich dude thing (though, who knows, there are a lot of future engineers/doctors around).
back to top 

GlaxoSmithKline plc, a research-based pharmaceutical company, recently announced the U.S. Food and Drug Administration’s approval of GSK’s FluLaval Quadrivalent influenza virus vaccine for individuals three years of age and older.
The vaccine is the second GSK intramuscular quadrivalent influenza vaccine approved by the FDA, following the approval in December of GSK’s Fluarix Quadrivalent. FluLaval Quadrivalent protects against two influenza A strains and two influenza B strains. Previous vaccines only included three strains to protect against two A virus strains and one B strain.
back to top 

Howard County is teaming up with Johns Hopkins University to launch a new accelerator geared toward commercial technology.
The new accelerator, called the Accelerator for the Commercialization of Technology, will help bring to market technology developed at the massive Hopkins Applied Physics Laboratory in Laurel. Howard County Executive Ken Ulman plans to formally announce the new partnership between the laboratory and the Howard County Economic Development Authority at an event Thursday.
back to top 

The Office of Technology Transfer (OTT), Office of the Director (OD), the National Institutes of Health (NIH), invites industry organizations (including corporations, partnerships, limited partnerships, and industrial development organizations); public and private foundations and nonprofit organizations to solicit research proposals from scientists across the NIH Intramural Research Program (IRP) for multiple focused research projects under a the NIH Cooperative Research And Development Agreement (CRADA) Program. This CRADA Program is an extension of collaboration opportunities solicited by NIH or developed on a one-on-one basis. As such, it is consistent with PHS Technology Transfer policy and the public health mission of the NIH. These collaboration opportunities are structured under the authority of 15 U.S.C. 3710a—Cooperative Research and Development Agreements. Note that the CRADA mechanism does not permit the transfer of funds from the NIH to a collaborator but does permit the collaborator to provide funding to the NIH researcher.
back to top 
by Brian Darmody
 The Chronicle of Higher Education reported release of the 2013 Academic Ranking of World Universities (ARWU) and it has good news for Maryland. The ARWU in the past decade has presented the Top 500 universities in the world ranked on quality of scientific papers and other methodologies to evaluate institutions of higher education. The Chronicle of Higher Education reported release of the 2013 Academic Ranking of World Universities (ARWU) and it has good news for Maryland. The ARWU in the past decade has presented the Top 500 universities in the world ranked on quality of scientific papers and other methodologies to evaluate institutions of higher education.
Harvard was ranked #1, but Johns Hopkins University was ranked number 17 in the world (#15 in US) and University of Maryland College Park was ranked number 38 in the world (#29 in US).
Having two highly ranked research universities in the relatively small state of Maryland, adjacent to our Nation’s Capital, is testament to the long term vision of our elected and university leaders in supporting research and higher education.
Many countries are working on strategies to have their universities enter the top 100 list. Maryland is fortunate to already be there, and we need to continue to build robust federal/university/corporate partnerships to continue and grow our state’s higher education research ecosystem.
ARWU Rankings: http://www.shanghairanking.com/Academic-Ranking-of-World-Universities-2013-Press-Release.html
back to top 
 Washington: A group of researchers have made a significant breakthrough for figuring out which mutations are benign and which are deleterious in cystic fibrosis. Washington: A group of researchers have made a significant breakthrough for figuring out which mutations are benign and which are deleterious in cystic fibrosis.
back to top 
 BioMaryland is supporting various events in France which benefit the biohealth community. See if your company qualifies for a travel assistance grant to fly out and attend! BioMaryland is supporting various events in France which benefit the biohealth community. See if your company qualifies for a travel assistance grant to fly out and attend!
back to top 

Biomedical engineers from The Johns Hopkins University have partnered with clinicians to create new therapeutic eye injections for a type of central vision loss caused by blood vessel growth at the back of the eye.
The new drug, with a biodegradable time-release coating is currently being tested to evaluate effectiveness in stopping such growth in mice.
back to top 

Investigators discuss new findings in Biomedicine and Biomedical Engineering. According to news reporting out of Baltimore, Maryland, by NewsRx editors, research stated, “Case reports document successful use of a high-density polytetrafluorethylene membrane to augment horizontal defects associated with immediately placed implants.”
Our news journalists obtained a quote from the research from the University of Maryland, “This membrane, which is designed to withstand exposure (not require primary closure) to the oral cavity because it is impervious to bacteria, reduces the need for advanced flap management to attain primary closure. Thus, the surgical aspect is less complex and the mucogingival architecture of the area can be maintained.”
back to top 

Hospital executives have never been frivolous when it comes to investing in technology, but as reimbursements shrink, the need to carefully analyze each purchasing decision has never been more urgent.
Given all the worthwhile – and not so worthwhile – options, what choices are hospital administrators currently making?
Since IT spending is largely taken up by meeting meaningful use and ICD-10 requirements, said Chantal Worzala, director of policy at the American Hospital Association, hospitals don’t have much left over for investments in other things.
back to top 
 Take Care Team Connect, an Evanston, Ill.-based IT firm that provides software platforms for population healthcare management while also offering provider coaching services on how to manage population-based workflows effectively. Take Care Team Connect, an Evanston, Ill.-based IT firm that provides software platforms for population healthcare management while also offering provider coaching services on how to manage population-based workflows effectively.
back to top 

Punit Dhillon built his career arranging Canadian venture capital funding for life sciences research and running companies that performed clinical trials in Canada.
Yet when he co-founded a company to develop cancer treatments two years ago, the president and chief executive officer of San Diego-based OncoSec Medical Inc. found he had no option but to operate solely in the United States.
back to top 

American healthcare workers’ confidence levels remained fairly consistent in the second quarter of 2013, according to the Q2 Randstad Healthcare Employee Confidence Index. Confidence levels among healthcare workers decreased by one-fifth of a point, to 54.3, in the second quarter of 2013.
Harris Interactive conducted the online survey o behalf of Randstad Healthcare in April, May and June of this year, among 188 healthcare workers, ages 18 and older. It included physicians, healthcare administrators, healthcare IT professionals and other healthcare professionals.
back to top 

What if we could increase productivity and stave the capital flight by helping Life Sciences startups build their companies more efficiently?
We’re going to test this hypothesis by teaching a Lean LaunchPad class for Life Sciences and Health Care (therapeutics, diagnostics, devices and digital health) this October at UCSF with a team of veteran venture capitalists.
back to top 
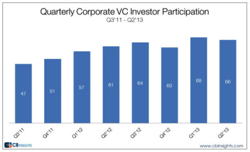
Corporate venture capital may be what fills in the large gaps VC has left behind, with CVC deals about 60 percent larger than VC deals on the whole.Healthcare corporate venture capital deals are up nearly 40 percent compared to Q1 and funding is up 34 percent, according to a report from CB Insights.
There were 126 CVC deals in Q2, ringing in at a combined value of $1.7 billion. Of these deals, 40 percent were at Seed or Series A-level, meaning CVCs may be willing to take risks VCs aren’t any more. According to the report, CVCs may help companies escape the Series A crunch: “One of every 4 CVC deals in Q2’13 were Series A deals ’ this represented an 800 basis point increase in Series A deal share vs. Q2’12.”
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 11
William E. Hanna Jr. Innovation Center at Shady Grove

September 12
Novartis Institutes for Biomedical Research

September 18-19
South San Francisco near the Airport
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 Do you want to help change the face of the Maryland Biohealth Community? Are you an experienced entrepreneur itching to work with disruptive technologies and innovative individuals to push them towards being the next big startup? Do you want to help change the face of the Maryland Biohealth Community? Are you an experienced entrepreneur itching to work with disruptive technologies and innovative individuals to push them towards being the next big startup?
BioHealth Innovation, Maryland’s youngest innovation intermediary wants to put you in the right place to make this happen. We need an experienced individual to to lead our Health IT initiative as our Health IT Entrepreneur-in-Residence (EIR).
back to top 
 Through a career spanning three decades and counting, Douglas Liu has managed various operations for life sciences companies in Boston, Chicago, Europe and Montgomery County. He has been involved with numerous organizations in the field, including the Tech Council of Maryland and the Governor’s International Advisory Council. Through a career spanning three decades and counting, Douglas Liu has managed various operations for life sciences companies in Boston, Chicago, Europe and Montgomery County. He has been involved with numerous organizations in the field, including the Tech Council of Maryland and the Governor’s International Advisory Council.
Such wide experience is a key reason he was recently chosen as board chairman of BioHealth Innovation, a Rockville-based public-private nonprofit that helps commercialize innovative ideas in the field and expand those companies. The partnership formed about two years ago after it was among the recommendations of the Montgomery County Biosciences Task Force.
back to top 

While scientists have struggled for decades to produce an effective malaria vaccine, Rockville-based biotech company Sanaria is encouraged enough by clinical trials that it hopes to have its malaria vaccine on the market in three to five years, The Gazette reports.
The results of a Phase I clinical trial of the vaccine—published Thursday in the online issue of Science magazine—show that the vaccine provided complete protection against malaria in subjects who were exposed to malaria parasites, according to a news release from Sanaria.
back to top 

Rockville-based Emergent BioSolutions Inc. on Friday announced the appointment of Barry Labinger, a well-known former executive at Human Genome Sciences Inc., to head its biosciences division.
Labinger served as HGS’ chief commercial officer from 2005 until last year, when GlaxoSmithKline plc snapped up the Rockville drug-maker for $3.6 billion. Glaxo made a clean sweep of Human Genome executives, including CEO Tom Watkins, shortly after.
back to top 

Through the Advanced Studies in Technology Transfer program, students will simultaneously gain the necessary knowledge and build professional networks with respected practitioners of the field. Our objective is to serve not only the needs of scientists and engineers who wish to pursue this non-traditional career, but also those of professionals who seek additional training.
View available courses in the Advanced Studies in Technology Transfer program.
More Information
The relatively new field of Technology Transfer can trace its origins and rapid growth to the economic developments and legislation of the early 1980s, a time when the US was looking to enhance its global competitiveness. While countries like Germany and Japan were exceptionally good at translating the ideas that originated from academic labs into useful products, US academic research results were by and large relegated to mere publications in scholarly journals. Concerned about the non-use of this potential goldmine of ideas, the US lawmakers passed a series of legislation in the early 1980s culminating in the famous Bayh-Dole Act. This Act has shifted the onus of commercialization from a central granting agency to the numerous grantees that receive the research funds, and the grantees have taken very enthusiastically to this shift. The Economist has lauded this Bill as the most inspired piece of legislation in the last half century.
back to top 

Thursday, August 22, 2013 10:00 am
This webinar, hosted by GUIRR, will present a new resource, the Federal Laboratory Consortium (FLC) Available Technologies tool. The tool provides a free, one-stop shop to locate licensing opportunities for a particular technology anywhere within the FLC’s nationwide system of federal labs and research centers. Fully equipped with Google’s advanced search capabilities, the FLC Available Technologies tool yields user-friendly results that can be saved, printed, or downloaded as a PDF with active hyperlinks that directly link to the featured technology.
Industry and academic representatives who would like to find out more about how this tool can reduce the time, effort, and guesswork needed to find federal laboratory inventions available to transfer are encouraged to participate.
back to top 
Our partner, the Economic Alliance of Greater Baltimore (EAGB) is in the running for the Economic Development Organization of the Year. The award is presented by Baltimore Innovation Week, a celebration of technology and innovation in Baltimore.
Help EAGB reach the number 1 spot by heading over to the Awards Site and and selecting Economic Alliance of Greater Baltimore in category 4, Economic Development Organization of the Year.


Scroll Down and Click


back to top 

With the global tuberculosis epidemic becoming more deadly, costly, and difficult to treat, Chinese Center for Disease Control and Prevention (China CDC) and Aeras today signed a memorandum of understanding to advance research and development of new tuberculosis vaccines. An improved TB vaccine offers the best hope for eliminating this airborne infectious disease that kills 1.4 million people worldwide each year.
While China has achieved significant reductions in TB illness and death over the past 30 years, TB remains a major public threat, with over one million new cases in China each year. A study published in the New England Journal of Medicine last year found that one in 10 cases of TB in China are resistant to the most commonly-used drugs. Based on the World Health Organization’s estimates of global multidrug-resistant TB (MDR-TB), China has the highest annual number of cases of MDR-TB in the world—a quarter of the cases worldwide.
back to top 

Let’s Celebrate How Baltimore Is Innovating.
The people and organizations represented below are advancing the city in new and inspiring ways, by growing businesses, increasing regional economic activity, publishing high-quality works of media, creating new gateways to the technology community and much, much more. They’re the best of the best in this community. This is the Baltimore region’s only people’s choice award for technology, entrepreneurship and new thinking.
Winners will be determined by online voting counts. Voting closes on Friday, September 13th. Awards will be announced at the Baltimore Innovation Week week closing party on Fri. Sept. 27.
back to top 

CareFirst BlueCross BlueShield has jumped head-first into the deep waters of mobile health. The health insurance giant has selected Cognizant, a mobile communication company, to provide a platform for getting mobile apps to its 3.3 million members.
Cognizant will help CareFirst offer its members “quick, secure and convenient ways” to manage their health coverage, access information about physicians and claims, and get information about benefits anytime, on any device, according to Cognizant officials in a news release.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Request for Applications (RFA):
Program Announcement (PA):
back to top 

The University of Maryland College Park, which likes to tout itself as a leading public research institution, has added a little more green to its laurels.
Sierra Magazine ranks the state’s flagship campus as the 13th greenest university in its latest national rating of “Cool Schools.”
UM trailed the University of Connecticut, Dickinson College, Stanford and American universities, among others, while besting the likes of Harvard, Yale and the University of California, Berkeley.
back to top 

The FDA has stamped an OK on GlaxoSmithKline’s HIV drug dolutegravir–to be sold as Tivicay. The approval marks another key advance for Glaxo ($GSK) on the regulatory front this year, providing a blockbuster candidate that analysts believe should do very well in competing against Gilead’s ($GILD) rival therapies.
“HIV-infected individuals require treatment regimens personalized to fit their condition and their needs,” said Edward Cox, M.D., M.P.H., director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research, in a release. “The approval of new drugs like Tivicay that add to the existing options remains a priority for the FDA.”
back to top 

This conference brings together small businesses, angel investors, venture capitalists, strategic partners, and business leaders from the biotech and pharmaceutical industries. It will feature presentations by top NHLBI SBIR- funded companies with innovative technologies on the brink of commercialization, an expert panel of investors, and opportunities for partnering and networking. Information about the NHLBI Office of Translational Alliances and Coordination, changes in the SBIR/STTR program re-authorization, and other funding opportunities and resources will be presented. NHLBI staff will be available to provide advice to applicants and awardees.
The NHLBI provides global leadership for research, training, and education to promote the prevention and treatment of heart, lung, blood, and sleep diseases and disorders and to enhance the health of all individuals so that they can live longer and more fulfilling lives.
back to top 

HHS is working to attract entrepreneurial talent to create a culture that supports risk-taking and accelerates innovation.
HHSentrepreneurs is based on the HHS Innovation Fellows Program launched in 2012. HHSentrepreneurs builds on lessons learned during the pilot HHS Innovation Fellows Program, and will:
- Identify HHS staff who are working on some of the Department’s toughest challenges and
- Match these talented individuals with External Entrepreneurs for a period of 6-12 months.
HHSentrepreneurs provides a novel framework to attract outside talent in areas including open innovation, agile development, and lean methodologies to accelerate innovation within HHS. Startup organizations have demonstrated that rapid iteration between various versions or features of a product can yield successful results, and HHS would like to promote these methods internally to address high-priority projects.
back to top 

A week after Cleveland’s nascent biotech accelerator BioMotiv bumped its financial reserves to $46 million, Torrey Pines Investment has stepped in to offer a $20 million commitment and close ties to the Russian drug development industry to back a joint effort to spawn new translational drug efforts around the globe.
In the pact, Torrey Pines Investment will work with its connections in the Russian and the Ukrainian pharma industry to facilitate the R&D networks that BioMotiv is developing. Far from the mainstream of the biotech industry’s global hubs, BioMotiv has been piecing together an effort to get new biomedical advances in academia into the clinic, positioning the programs for an out-licensing deal with a biotech or pharma company that can take them the remaining distance to a potential regulatory approval. Nationwide Mutual Insurance Company and several individual investors have now bumped its capital reserves as BioMotiv scouts for additional capital to complete its fundraising effort.
back to top 
In the middle of 2003, Marc LaForce was having trouble sleeping. As the director of the Meningitis Vaccine Project (MVP), he was missing a vital piece of a difficult puzzle. The MVP sought to commercialize a vaccine that would help prevent Africa’s devastating epidemics of meningococcal meningitis, a bacterial infection of the brain and spinal cord. The largest documented outbreak, in 1996, sickened 250,000 people in Africa and caused 25,000 deaths. In 2002, a single West African country (Burkina Faso) had 13,000 meningitis cases and at least 1,500 deaths.
To succeed, the MVP needed a vaccine production method with two essential qualities: very effective and very affordable. But in mid-2003, that search hit a dead end. “All projects have their ups and downs,” says LaForce, M.D. “We were in the downest of downs.”
back to top 

This week, FDA issued final guidance on incorporating and integrating radio frequency wireless technology into medical devices, Bloomberg BNA reports.
In a blog post on Tuesday, FDA Senior Policy Adviser Bakul Patel wrote, “Our goal is to help industry develop a range of innovative, safe and effective medical devices that incorporate wireless technology, which can, in turn, help reduce health care costs, enhance quality and benefit patients and providers alike” (Weixel, Bloomberg BNA, 8/14).
back to top 

The new landscape for venture capital investing does not seem to leave much room for classic company formation. Investor after investor has shut down or moved beyond startups into what seem like greener pastures.
So it should come as no surprise that at least a few VC firms are now expanding into the royalty space, as shown by a deal announced this week. Aisling Capital and Clarus Ventures, two top-tier VC firms, acquired 20 percent of the royalty stream created by sales of ibrutinib, a novel tyrosine kinase inhibitor developed by Pharmacyclics (NASDAQ: PCYC) and partnered with Johnson & Johnson (NYSE: JNJ) for use in B-cell malignancies such as chronic lymphocytic leukemia (CLL).
back to top 

DreamIt Health Demo Day has gotten a lot of great press, from Philly.com to NewsWorks to Technical.ly Philly, and many more. These plaudits are well deserved: DreamIt Health was the first Philadelphia-based health care accelerator, and by all accounts it was a huge success. Ten health care startup companies participated in this venture, made possible by a collaboration between Independence Blue Cross (IBC), Penn Medicine, and DreamIt Ventures. The more than 300 attendees to the Demo Day on July 24, 2013 included potential investors and customers, mentors, and health care executives.
We at Wharton Entrepreneurship are delighted, and not only because this event was such a terrific demonstration of the robust community of heath care entrepreneurs in the Philly region. We’re proud to point out that the founders of four of the presenting companies and both of the speakers are all Wharton Entrepreneurship alumni, several of whom went through our Venture Initiation Program (VIP)! If that’s not a demonstration of Knowledge in Action, then I don’t know what is.
back to top 

Due to the global shortage of donor hearts, patients on the waiting list for a heart transplant can wait several months to several years for a match to be found. But for 36-year-old Frédéric Thiollet, who surpassed two years of support with the SynCardia temporary Total Artificial Heart on Aug. 5, he’s enjoying each and every day.
“I feel well and I am confident, having been implanted now for over two years,” said Frédéric Thiollet. “I have recuperated all my physical functions, even my sexual activity, which I had believed was gone forever. In my own words, I have enjoyed an effective resurrection, a new birth. Physically I have no limit. I am as strong and powerful as before, even more so than before.”
back to top 

BioBoost, a consortium of OrbiMed Advisors LLC, Johnson and Johnson (NYSE: JNJ) and Takeda Pharmaceutical Co. Ltd. (TSE: 4502) has won the tender to establish the life sciences incubator as part of the Office of the Chief Scientist’s incubator program.
The franchise for the biotech tender, like the franchise for all incubators since the reform, is for eight years. The consortium members will receive NIS 6.9 million in financing over three years, more than franchisees of incubators in other fields, as part of the government’s wish to provide special support for the life sciences. Most of the incubator’s companies will develop drugs, and a few will develop medical devices.
back to top 
University of Maryland researchers announced last week they have been able to successfully turn a fish known for eating anything, the cobia, into a vegetarian.
While altering any animal’s diet may seem unnatural or even wrong, the biologists said the development could mean big bucks for the seafood industry.
“Aquaculture isn’t sustainable because it takes more fish to feed fish than are being produced,” said Aaron Watson, a graduate assistant at the university’s Center for Environmental Science. “But a new vegetarian diet might change everything.”
back to top 

XLerate Health, a Louisville-based accelerator for early-stage, health-related companies, has selected six companies to participate in its inaugural class. The accelerator’s 10-week intensive immersion program began Monday and runs until “Demo Day” on Oct. 25.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9-10
Boston Park Plaza Hotel & Towers

September 9-27

September 12
Novartis Institutes for Biomedical Research

September 18-19
South San Francisco near the Airport

October 11
Georgetown Conference Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
| | | | | | | | | |