|
|
|

Baltimore-based Cerecor has gathered up the first half of a $33 million B round, with plans to devote much of that money to a midstage development program for an experimental depression therapy recently punted by a restructuring Merck ($MRK).
In documents filed with the SEC, the biotech noted that it has raised $16.2 million of a planned $33.2 million round. New Enterprise Associates, Apple Tree Partners and MPM Capital led the round, which is also going to fund R&D efforts on its catechol-O-methyltransferase inhibitor platform for conditions “characterized by impairment of executive function and working memory.”
back to top 

Well, this is interesting.
RockThePost, a site that connects startups and investors, just announced that it has merged with CoFoundersLab, a site that helps entrepreneurs find cofounders.
RockThePost, which launched in 2011, sought to form a new kind of social network that brought together money-hungry startups with equity-hungry investors. Although the site never became a household name, its model proved somewhat successful: CoFoundersLab actually raised a portion of its initial $750,000 seed round on RockThePost.
back to top 

DED awarded a Montgomery County Economic Development Fund grant providing up to $12 million in property tax incentives for customers of BYTEGRID®, a leading data center company located in Silver Spring that is focused on the nationwide acquisition, development and operation of premier multi-tenant data center facilities.
“Montgomery County is focused on increasing our attractiveness to large private and public enterprise organizations seeking to optimize costs by outsourcing IT infrastructure,” said Steve Silverman, Director of Economic Development for Montgomery County. “This strategic grant for BYTEGRID’s data center customers shows our commitment to fostering a pro-business climate that already includes leading digital age businesses in healthcare, financial services, IT services and cyber security. The key takeaway here is that Montgomery County, Maryland is in the regional and national big data game to win.”
back to top 
NexImmune, an early stage biopharmaceutical company, today announced it has secured $3 million USD in financing led by New Enterprise Associates (NEA). Other investors include Pfizer Venture Investments and Amgen Ventures. In association with the financing, Jim Barrett of NEA and Janis Naeve of Amgen Ventures were named to NexImmune’s board of directors.
NexImmune is developing immunotherapy products from its proprietary Artificial IMmune (AIM™) nanotechnology. The AIM technology, which was first developed by Drs. Mathias Oelke and Jonathan Schneck at The Johns Hopkins University, has several potential applications for the treatment of cancer, autoimmune disorders, and infectious disease. Proceeds from this financing will be used to support pre-clinical development of the company’s first product, AIM 101, an artificial Antigen Presenting Cell (aAPC) for the treatment of cancer.
back to top 

Analytical Informatics, Inc, a portfolio company of the New Ventures Department of the University of Maryland, Baltimore is one of the semifinalists in 43North, the world’s largest business idea competition. 43North will award $5 million in cash prizes and is setting out to turn the best new business ideas from around the globe into reality. The top award is $1 million, with six $500,000 awards and four $250,000 awards. There were with 6,932 applications from 96 countries and all 50 U.S. states. That got boiled down to 2,603 qualified submissions and 113 semifinalists. Analytical Informatics, Inc was the only Baltimore and Maryland based company selected.
back to top 

Promising news for any unlucky american in immediate need of a doctor: BetterDoctor, the San Francisco based healthcare tool and online doctor search service, has closed a $10 million Series A funding round led by New Enterprise Associates and participated by existing investors including SoftTechVC and Finnish venture capital firm Lifeline Ventures.
The fund will be used to further develop the existing service, hire new employees and spread the service’s web and mobile applications to new platforms.
back to top 

Sen. Barbara Mikulski toured the MedImmune laboratories in Frederick on Monday morning and talked with employees.
Mikulski told the workers she was proud of what they are doing, creating medical solutions to health problems. She also talked with company executives about the company’s future.
back to top 

Qiagen N.V,, the leading global provider of sample & assay technologies that are used to transform biological materials into valuable molecular information, has launched 14 new GeneRead DNAseq V2 gene panels targeting an extensive range of cancer-related genes or gene regions. Gene panels are an integral part of many next-generation sequencing (NGS) workflows. They are used to enrich genes of interest in a sample prior to sequencing runs and as such can be seen as the core assay in the workflow.
Qiagen’s new GeneRead panels are customisable to include other genes or gene regions of clinical or biological interest and are compatible with any NGS sequencer. They are part of Qiagen’s industry-leading sample-to-library workflows, which are helping to drive the growing use of NGS in clinical applications.
back to top 

Severe bleeding is a primary cause of death for soldiers wounded on the battlefield. Deep shrapnel and gunshot wounds can be notoriously difficult to control, particularly in soft tissue places such as the neck, shoulder, and groin. Now undergrad students at Johns Hopkins University have developed a prototype device to help address such trauma in a speedy fashion.
The new device is a large plastic syringe with two compartments, similar to epoxy injectors, holding a polyol and a diisocyanat, chemicals that produce polyurethane foam when mixed. The foam hardens shortly after injection and delivers pressure to the wound from within. The hope is that the foam will help soldiers from bleeding out while they’re on the way to a medical facility where a surgeon can address the wound directly.
back to top 

Pharmaceutical giant AstraZeneca has revealed proposed designs for its new corporate headquarters and global research centre in Cambridge, which is scheduled to open in late 2016.
The plans for the new facility, which will be located on the Cambridge Biomedical Campus (CBC), include designs for the global centre, an R&D enabling building and an energy centre.
back to top 

Maryland has a future in being the perennial powerhouse in cyber security but it’s lacking something that will help the state’s industry take off like a rocket — and that’s depth.
In Friday’s print edition cover story I wrote about how colleges and universities are scrambling to change curriculum to prepare more students for success in the cyber world post graduation. Industry executives have lamented that colleges aren’t doing enough to prepare students to fill vacant positions — more than 10,000 across Maryland in 2013, according to career services firm Burning Glass.
back to top 

The head of a young Baltimore cyber security company says a New York investor nixed a deal after discovering Maryland’s new cyber security tax credit wouldn’t transfer to investors.
Suzanne Magee, CEO of Bandura LLC, said the credit should go to the investor. That’s how it works with the state’s biotech tax credit.
back to top 

Baltimore-area companies took home 12 percent of the money venture firms invested in the Baltimore-Washington, D.C., region in the second quarter.
Greater Baltimore companies raised a total of $29.5 million in venture capital during the period, according to a MoneyTree Report by PricewaterhouseCoopers and the National Venture Capital Association. Venture capitalists invested a total of $247.3 million in 58 companies in Baltimore and D.C. during the quarter.
back to top 

While the University of Virginia and Johns Hopkins can’t exactly compete with Ivy League Harvard when it comes to dominating the ranking of college rankings, the two D.C. area schools were ranked among the top 50 universities in the world by the Center for World University Rankings. Crushing other top-tier institutions like Dartmouth, Brown and McGill, the local academic titans nourishing the minds of students across the globe substantiated themselves as schools worth attending during a time of loan shark annihilation.
back to top 
President of the University of Maryland-Baltimore County since 1992, Freeman A.  Hrabowski III thinks he knows what students need: lots of support. Morally, colleges owe it to students to do everything possible to help them succeed, he said in a recent visit to The Chronicle, and a higher retention rate means more tuition dollars, too. Hrabowski III thinks he knows what students need: lots of support. Morally, colleges owe it to students to do everything possible to help them succeed, he said in a recent visit to The Chronicle, and a higher retention rate means more tuition dollars, too.
back to top 

Former state economic development secretary Aris Melissaratos is leaving Johns Hopkins University for a job where he’ll have more power.
Melissaratos, who had been with Hopkins for seven years, on July 17 joined Stevenson University as interim dean of the Brown School of Business and Leadership. Melissaratos said he has always had an interest in academia and now will be able to play a leading role in preparing students to join the local workforce.
back to top 

Venture investors in Silicon Valley are frequent prey of startup founders who spot them in public places — and semi-public places — and start pitching. Last night, a discussion about awkward pitches led four VCs on a panel for the Silicon Valley Business Journal’s annual Pitch event to tell tales from the mens room.
The consensus? There’s no good way to do it.
back to top 

That was my question when I spoke with DreamIt Health newbie BioBots, specifically co-founder Ricardo Solorzano. He has spent three years working on technology to develop low-cost 3D bioprinters that research scientists can use to develop biomaterials. The company was formed by a couple of University of Pennsylvania graduates They view it as a way to change the way people think of regenerative medicine.
Solorzano and fellow co-founder Danny Cabrera joined Hive 76 in Philadelphia so they could learn more about developing these 3D printers. They also work with intern Eric Wamakima. They spoke with MedCity News at DreamIt Health’s launch party for its second class
back to top 

It’s tough for medical device companies to get that stamp of FDA approval, but the labyrinthine regulatory pathway certainly doesn’t help things. Regulators want to change that:
“…we learned that the delivery of new therapies to patients can be accelerated if medical device innovators — including entrepreneurs and university students and faculty — understand FDA’s regulatory processes,” FDA researcher Francis Kalush wrote recently.
back to top 

President Obama’s visit earlier this month to 1776, the startup hub in downtown Washington, D.C., shined a spotlight on entrepreneurship and innovative thinking nationwide. It was a good location because the contemporary, well-lit loft that incubates and supports area startups has grown from zero to 215 members in only a year-and-a-half.
The president’s visit highlighted two entrepreneurial stories, not only of 1776 but also of the early steps by American University involving its Masters of Arts in Media Entrepreneurship program (MAME) and two campus collaborators. We became the hub’s first university partnership in a fruitful relationship that could be repeated at other campuses across the country.
back to top 

The two-year anniversary of the monumental Supreme Court decision upholding the Affordable Care Act may not have caused much fanfare, but Steve Kraus, a partner at Bessemer Venture Partners, said Massachusetts should be celebrating.
Here are four takeaways from a conversation with the head of the firm’s health care investing, as he analyzes the shifts that have occurred in the two years since the Affordable Care Act was deemed constitutional.
back to top 

Many medical researchers with ideas not tied to a drug backed by the pharmaceutical industry often find themselves without funding; patients with diseases or other medical conditions, particularly those that are rare, are without much power. That is, until recently.
Now, scientists are turning to crowdfunding to launch their research. Donors won’t get a free CD, T-shirt or other giveaway like they do on Kickstarter. But what fundraisers give in return is hope for a treatment and at least insight into medical conditions.
back to top 

Paul Thompson, director of IT innovation at Medtronic, explained how he held on for four years to bring the “hospital of the future” to life. His work is part of the company’s larger push to move from a device maker to a healthcare services provider, which he compared to IBM’s move from hardware to services.
At the end of this session at CONVERGE, an audience member asked what metrics Thompson and his team must meet each year to be considered a success.
back to top 

DreamIt Ventures is kicking off its second health IT accelerator class in Philadelphia with a stronger life sciences theme than last year. More hospitals reviewed candidates this year and the result included four life sciences startups in its nine-member class. They take diverse approaches to the problems they solve — from molecular diagnostics to detect disease earlier to wound management.
It’s an interesting development because healthcare startup accelerators tend to stick to health IT and mobile health companies because it takes a shorter amount of time to develop products, there’s less risk and the path to getting FDA clearance can take a while. Although it’s not interested in therapeutics, DreamIt has definitely been warming up to medical technology that goes beyond health IT. One reason is that molecular diagnostics and 3D printing is increasingly accessible through mobile platforms.
back to top 

Stanford University scientists say they have developed a new test for type 1 diabetes that will cost a fraction of the current price and could speed up diagnosis from days to hours. That could be useful anywhere, but especially in poorer countries where many people with diabetes go undiagnosed or misdiagnosed because the existing tests are too expensive to be widely offered.
In current tests, blood samples are sent to a lab, where radioactive materials are used to detect the cause of the disease: a so-called auto-antibody that attacks the insulin-producing cells in the pancreas. This test is labor-intensive and costs hundreds of dollars.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 14
1776

August 14
Smokey Glen Farm Barbequers

September 15-16
Sheraton Pentagon City

September 15
Various Locations
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Supernus Pharmaceuticals, Inc. (Nasdaq:SUPN), today announced the execution of a royalty acquisition agreement (“Agreement”) with HealthCare Royalty Partners (“HC Royalty”). Per the Agreement, HC Royalty will make a $30 million cash payment to Supernus in consideration for acquiring from Supernus certain royalty and milestone rights related to the commercialization of Orenitram(TM) (treprostinil) Extended-Release Tablets by Supernus’ partner United Therapeutics Corporation. Supernus will retain full ownership of the Royalty Rights after a certain threshold has been reached per the terms of the Agreement.
“We are pleased to have completed this royalty transaction, which strengthens our balance sheet and enhances our financial flexibility,” said Jack Khattar, President and Chief Executive Officer of Supernus. “The transaction allows us to partially monetize our royalty stream from Orenitram(TM) for a significant cash consideration while positioning Supernus to further benefit from the future upside potential of the product.”
back to top 

Healthcare Interactive Inc. — which sells Web-based software for companies to monitor medical costs in real time — has raised $8 million in funding.
The Series A funding in the Glenwood, Maryland-based company came from Grotech Ventures, which has more than $1.3 billion under management, and Harbert Venture Partners, an emerging growth-stage investor with $200 million under management.
back to top 

ORTHOMETRIX, INC. announced today that it has signed an exclusive agreement with ENCORE PATH, INC. to market, sell and service worldwide the Tailwind™ arm rehabilitation device. The Tailwind™ is a patented Bilateral Arm Trainer with Rhythmic Auditory Cueing (BATRAC) that was developed at the University of Maryland School of Medicine’s Department of Physical Therapy and Rehabilitation Services and licensed to Encore Path, Inc., a portfolio company of the New Ventures Department of the University of Maryland, Baltimore. The Tailwind has been shown to improve arm movement in stroke patients with paralysis. It will be initially marketed in Asia and in Israel, and then in the U.S. after a Centers for Medicare & Medicaid Services (CMS) HCPCS code is obtained for use of the Tailwind as a durable medical equipment (DME). Commenting on this agreement, Reynald Bonmati, Chairman and Chief Executive Officer of Orthometrix, stated, “I am very pleased to partner with Encore Path and the University of Maryland. The Tailwind™ for upper-limb rehabilitation of stroke patients is a natural addition to our SmartStep® Biofeedback system for lower-limb rehabilitation, developed and manufactured by our partner Andante Medical Devices, Inc. Orthometrix, Andante and Encore Path are currently working on the manufacturing of the Tailwind™ by Andante.
The Tailwind was developed by physicians from the University of Maryland specializing in physical therapy and rehabilitation after a decade of scientific research. Clinical studies have shown that the device helps improve arm mobility, function, and range of motion in patients with even severe paralysis. Representatives from the University of Maryland’s New Ventures Department facilitated the introduction of Encore Path, Inc. and Orthometrix, Inc. and the subsequent agreement. The University of Maryland Ventures is designed to fuel the growth of the University’s startups, particularly those based upon intellectual property developed by physicians at the University of Maryland, Baltimore and University of Maryland, College Park.
back to top 

Storrer will oversee strategy, expansion and operation of Strand Genomics Inc., the wholly owned subsidiary of Strand Life Sciences in North America. Strand is a leader in technology innovations for personalized medicine using genomics in over 2,000 clinical and research institutions worldwide. By enhancing sequence-based diagnostics and clinical genomic data interpretation using a strong foundation of computational, scientific, and medical expertise Strand is bringing individualized medicine to the world.
“We are excited to have Scott Storrer join our team,” said Dr. Vijay Chandru, Chairman and CEO of Strand. “Storrer’s twenty plus years of executive experience leading and growing profitable businesses in the U.S. healthcare industry across payer, provider and personalized medicine sectors will help develop a strong presence for Strand in North America.”
back to top 

New Enterprise Associates averaged a U.S. funding deal every three days in the first half of this year, the hottest six months for the industry since the end of the dotcom boom in 2001.
Not far behind NEA’s 64 first half deals were Kleiner Perkins Caufield & Byers (54), Andreessen Horowitz (52) and Google Ventures (50), according to a report from investment database research firm CB Insights.
back to top 

Take advantage of an incredible international business opportunity and explore the India market with Montgomery County Executive Leggett during a business mission to India this November!
Sign up now to join Leggett and fellow business, educational and community leaders for engaging stops in New Delhi, Bangalore, Hyderabad and Raipour. August 1st is the deadline to apply for ExportMD grant funds to help defray the cost of participation in the m
back to top 

Johns Hopkins University is expected to receive almost $6.4 million from the federal health department for a support program for people with dementia. The award, worth up to $6.38 million, is part of the federal Centers for Medicare and Medicaid Services’ Health Care Innovation Awards.
The award is contingent on final administrative approval by the federal health department.
back to top 

Maryland has one of the Top 10 best-buy colleges and universities, according to the 2015 edition of Fisk’s Guide to Colleges.
The University of Maryland, Baltimore County ranked No. 9 out of 44 schools in the U.S., United Kingdom and Canada.
back to top 

The Frederick Innovative Technology Center Inc. is looking for business leaders to volunteer for its board of directors.
Preference will be given to those in private industry in the fields of life sciences, advance technology, finance, marketing and entrepreneur. The board meets the fourth Monday of each month.
back to top 

Alexandria-based venture capital firm Columbia Capital is planning a $425 million fund according to SEC filings reported in the Washington Business Journal. The firm invests in information technology, especially infrastructure, wireless spectrum and other related fields. It’s done plenty of investing around D.C. in the quarter century since it was founded. Millennial Media, Broadsoft, Virtustream and Summit IG are all on the list of local companies invested in.
Not that Columbia limits itself geographically. It led a $23 million round of funding for Seattle-based 2nd Watch in November. According to WBJ, it even occasionally builds a company from scratch to satisfy some IT need, like Cloud Sherpas, which provides enterprise cloud-based services. Whether or not that will be the path Columbia takes this time remains to be seen, but it’s certainly not out of the realm of possibility that the fund is being raised for creation rather than strictly investment.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
- NIH Announces Change in Policy Requirements for Activation Notices for Fellows Sponsored by Foreign and Federal Institutions
(NOT-OD-14-101) National Institutes of Health
- Notice of Correction to Budget Instructions for PA-14-042 “NIH Pathway to Independence Award (Parent K99/R00)”
(NOT-OD-14-102) National Institutes of Health
- Request for Information: Collaborative Translational Research Consortium to Develop T4 Translation of Evidence-based Interventions
(NOT-HL-14-028) National Heart, Lung, and Blood Institute
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Date: Monday, September 22, 2014
Location: North Carolina Biotechnology Center 15 TW Alexander Drive Research Triangle Park, NC 27709-3547 http://www.ncbiotech.org/
Background:
NHLBI’s Office of Translational Alliances and Coordination (OTAC) hosts this semi-annual Regional Innovation Conference that brings together small businesses, angel investors, venture capitalists, strategic partners, and business leaders from the biotech and pharmaceutical industries. NHLBI staff will be available to describe the details and impact of recent changes in the Federal SBIR/STTR program, as well as note other funding opportunities and resources for NHLBI small businesses.
Previous conferences have been held in Boston, MA, San Francisco, CA, San Diego, CA, and Rockville, MD.
back to top 
Founder salaries
Q.: Is it okay to pay yourself (and other founders) a salary when you’ve only raised money from friends and family? If so, how much is reasonable? What’s an acceptable salary to pay yourself once a non-family seed round is raised?
back to top 

The Tech Council of Maryland hopes a new office at the University of Maryland BioPark will help the organization play a bigger role in technology and life sciences in Baltimore.
Much of the tech council’s activities are centered in Montgomery County, where the organization’s headquarters and many of its members are based. The tech council will open a new office at the biopark and staff it every Thursday. The Baltimore office will focus on strengthening partnerships with other technology organizations in the city and developing new programs that meet needs of Baltimore-area companies.
back to top 

More than 70 percent of college graduates with a bachelor’s degree in science, technology, engineering and mathematics are not employed in those industries.
About 74 percent of STEM college graduates hold jobs unrelated to their fields of study, according to U.S. Census Bureau data. And those who are working in STEM fields are predominantly male. Specifically, Census data shows more than 80 percent of engineers and more than 70 percent of computer professionals are men.
back to top 

Happy 10th anniversary to the National Institutes of Health’s Common Fund! It’s hard to believe that it’s been a decade since I joined then-NIH Director Elias Zerhouni at the National Press Club to launch this trans-NIH effort to catalyze innovation and speed progress across many fields of biomedical research.
Allow me to take this opportunity to share just a bit of the history and a few of the many achievements of this bold new approach to the support of science.
back to top 

It’s still a good time to go public.
Twenty-eight venture-backed companies completed initial public offerings during the second quarter, raising $4.9 billion. That was a 45 percent increase in dollars raised compared to the previous quarter, according to an analysis by Thomson Reuters and the National Venture Capital Association. It was the fifth consecutive quarter of more than 20 IPOs at venture-backed companies.
back to top 

In biotech’s early days, telling a story to a wide audience used to be part of the path to success. Founders would share a compelling early narrative to potential investors, reporters, and just about anyone else who would listen. Nature papers were the coin of the realm.
But far from shouting to the rooftops, lately it seems that more and more biotechs are pursuing a different approach. Instead of keeping their technology under wraps until a first financing happens, these companies go into what we call “permanent stealth mode.” The principle here seems to be, “say no more publicly than necessary, and even then, keep it vague.” Meantime, let your actions speak for you: Raise money. Sign partnerships with pharmaceutical companies. And then, seemingly out of nowhere, hand consumers and investors a finished product or service.
back to top 

On a recent tour of the latest addition to University City Science Center’s campus in Philadelphia – an innovation hub in an established building on its campus set to officially open next week — I unexpectedly came across an office belonging to Merck.
It’s all the more interesting because it’s just a few doors down from DreamIt Ventures and its accelerator DreamIt Health, which has a second class of health IT entrepreneurs moving in next week. The Science Center’s own digital health accelerator will also be working in the building.
back to top 

The 4th edition of the Orphan Drugs Summit, Northern Europe’s premier orphan drugs conference, will take place in Copenhagen, Denmark this September.
As the conference approaches it is becoming more obvious that collaboration is one of the most important driving forces in the development of orphan drugs. The Orphan Drugs Summit will focus on how to build relationships on multiple levels, highlight how to access partnership parallel advice, and will also outline how stakeholders can benefit from collaboration to develop stronger clinical trials.
back to top 

Healthcare startups, and all startups for that matter, need investors and funding to stay alive. For this reason, MedCity is pleased to announce that investor Andrew Jay, MD, will be speaking at CONVERGE. Dr. Jay is the head of the medical solutions fund at HealthCare Fund at Siemens Venture Capital, which invests in companies focusing on imaging, diagnostics and health IT.
Jay will be speaking on the second day of MedCity CONVERGE at 9:20 a.m. The third annual CONVERGE is July 15-16 in Philadelphia.
back to top 

Two private equity firms announced the formation of a $26.6 million fund that will focus exclusively on the aging marketplace and companies within it.
Chicago-based Ziegler and Ohio-based Link-age Ventures announced the close of the Longevity Fund, which aims to address a number of key issues affecting seniors. Among them: coordination of care, chronic disease management, reducing hospitalizations and re-admissions, disease prevention and wellness, aging and government funded programs and public health issues, according to the companies.
back to top 
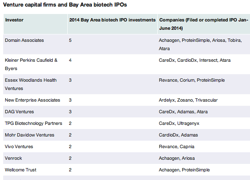
A cadre of venture capital firms could cash in from the wave of Bay Area biotech initial public offerings in the first half of the year.
Stalwart VCs such as Kleiner Perkins Caufield & Byers of Menlo Park and Domain Associates are among the venture capital firms whose names pop up most often in the IPOs of local life sciences companies through June. Among the other VCs are DAG Ventures of Palo Alto and Essex Woodlands Health Ventures in Palo Alto.
back to top 
Personalized medicine (PMx), medical treatment tailored to specific patient populations based on their genetic or molecular biology profiles, has long been heralded as the next big thing in healthcare. It’s been about 16 years since Genentech launched Herceptin, a drug for breast cancer patients with a specific genetic mutation. At the time, Herceptin seemed to usher in a revolution for how drugs would be developed and patients would be cured.
In that new version of care, drugs could be tailored to a patient’s specific biochemical profile, dramatically improving efficacy rates and reducing the system-wide costs and complications associated with one-size-fits-all medications. For pharmaceutical manufacturers, this approach had the potential to improve sales and profits through a radically new business model: differentiated products for segmented populations (see “A Strategist’s Guide to Personalized Medicine,” by Avi Kulkarni and Nelia Padilla McGreevy, s+b, Winter 2012).
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

July 17
Roof Bethesda

August 14
1776

August 14
Smokey Glen Farm Barbequers

September 15-16
Sheraton Pentagon City

September 15
Various Locations
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, today announced the launch of a new service offering: the Startup Package for Client Companies. The new package is designed to provide early-stage biohealth companies in Central Maryland with access to critical professional services – including corporate legal guidance, intellectual property, banking, accounting, payer and strategy, and grant support – as an enhanced benefit to working with BHI. BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, today announced the launch of a new service offering: the Startup Package for Client Companies. The new package is designed to provide early-stage biohealth companies in Central Maryland with access to critical professional services – including corporate legal guidance, intellectual property, banking, accounting, payer and strategy, and grant support – as an enhanced benefit to working with BHI.
“We are proud to offer this service to our startup clients seeking to commercialize biohealth products,” said Richard Bendis, President & CEO. “BHI aims to facilitate the development of commercially viable health-related products and companies. Providing access to these professional services is a critical step towards helping our biohealth startup clients in achieving this goal.”
BHI has partnered with a supporting network of professional service providers that share the same vision for growing Maryland’s biohealth sector, including Venable LLP, M&T Bank, Aronson, Miles & Stockbridge, InHouse Patent Counsel, Science Sherpa, Center for Medical Technology Policy, ADVI, and BBC Entrepreneurial Training & Consulting. These firms understand the nature and needs of startup companies, and offer preferred rates for client companies.
back to top 

Rockville biotechnology company Cellphire is the latest receipient of funding via the State’s InvestMaryland program, receiving $1 million last month!
Cellphire is developing stabilized cellular products, including freeze-dried platelets that can be stored for years and used in a range of advanced therapeutic and diagnostic applications including sports medicine, plastic surgery and dentistry. The company received a contract last year worth up to $57 million from the Biomedical Advanced Research Defense Authority, a division of U.S. Department of Health & Human Services. The InvestMaryland funding will be used to continue development of the company’s freeze-dried platelet product, Thrombosomes, and move it closer to winning FDA approval.
back to top 

GSK and Save the Children have announced the second annual $1 million Healthcare Innovation Award, which was established to identify and reward innovations in healthcare that have proven successful in reducing child deaths in developing countries.
Organizations from across the developing world can nominate examples of innovative healthcare approaches they have discovered or implemented. These approaches must have resulted in tangible improvements to under-5 child survival rates, be sustainable and have the potential to be scaled-up and replicated. Special attention will be given to work that aims to increase the quality of, or access to, healthcare for newborns.
back to top 

Take a break from the Summer heat with our sponsor, Hydro Service and Supplies, and join us for another great networking event on July 9th from 5:00 – 7:30 p.m. at American Tap Room in Rockville, MD. This location is a short walk from the Metro located in the Rockville Town Center.
Hydro Service and Supplies, Inc. provides a versatile range of quality ultrapure water systems and products, from large scale central production systems to point-of-use laboratory systems. Since 1967, Hydro has been an industry leader by combining synergistic engineering and innovative designs with high-performance component selections, superior materials of construction, and precision manufacturing that result in high quality ultrapure water systems.
Hydro is dedicated to providing quality products and professional support from our experienced sales, engineering and service teams. Hydro serves Pharmaceutical, Biotech, Microelectronic, Research, Academic, Medical/Clinical, Food and Beverage, and Industrial applications. In addition, Hydro offers engineering services, turn-key installation, start-up and commissioning, validation support, PLC programming, water testing services and reliable service support 24/7/365.
back to top 

Originating as a solution to educate health workers in developing countries, emocha is now a powerful platform that allows researchers and clinicians to use mobile data capture, health education, and communication to address the challenges of adherence, linkage to care, and patient data management across a myriad of use cases. Sebastian Seiguer, CEO and Founder of emocha, and Morad Elmi, Director of Marketing, spoke with us about the latest from this Baltimore-based startup.
back to top 

Two Montgomery County companies each took home the top prize of $100,000 in their category as part of the 2014 InvestMaryland Challenge.
Bethesda-based life sciences company, Brain Sentry, won for its helmet-mounted sensor used to identify team sport players who should be evaluated for a concussion. Gaithersburg-based IT company, ClickMedix, won its catergory for technology aimed at helping physicians and health organizations maximize the number of patients they are able to serve.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
- Notice of CDC/NCCDPHP Participation in PA-14-071 “PHS 2014-02 Omnibus Solicitation of the NIH, CDC, FDA and ACF for Small Business Innovation Research Grant Applications (Parent SBIR [R43/R44])”
(NOT-CD-14-001)
Centers for Disease Control and Prevention
National Center for Chronic Disease Prevention and Health Promotion
- Notice of Website for Frequently Asked Questions (FAQs) for PAR-13-231 “Phenotyping Embryonic Lethal Knockout Mice (R01)”
(NOT-HD-14-021)
Eunice Kennedy Shriver National Institute of Child Health and Human Development
Requests for Applications:
- Enriching the Hematology Research Workforce through Short-term Educational Experiences in Emerging Science Research Education Program Grant (R25)
(RFA-HL-15-006)
National Heart, Lung, and Blood Institute
Application Receipt Date(s): October 13, 2014
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Leslie Ford Weber, interim executive director of Johns Hopkins University’s Montgomery County Campus, has been appointed to a board of directors executive committee position with the Montgomery County Chamber of Commerce.
Weber will serve a yearlong term as vice chair of the economic development committee. She shares the responsibility with Stewart Edelstein, executive director at the Universities at Shady Grove.
back to top 

The Johns Hopkins University has joined the National Science Foundation‘s National Innovation Network and becomes the fourth member university in the NSF Innovation Corps regional collaboration led by the University of Maryland, along with the George Washington University and Virginia Tech.
The NSF has approved a request from the three original universities to officially include Johns Hopkins in the I-Corps program’s “node” in the Mid-Atlantic called DC I-Corps, which was formed last year with $3.75 million in NSF funding.
back to top 

University of Maryland, a national leader in entrepreneurship education and venture creation, announces it will offer a new master’s degree program in technology entrepreneurship starting this fall.
The 30-credit, 15-month Master of Technology Entrepreneurship, available online to current and aspiring entrepreneurs worldwide, features the university’s most advanced and comprehensive entrepreneurship curriculum to date, taking students from concept development and prototyping to business model generation and customer validation, as well as legal aspects of entrepreneurship, financial and innovation management, and effective growth strategies.
back to top 

Former Johns Hopkins University college student Alex Koren wants to make a difference.
Koren feels he has a better opportunity of doing that by dropping out of Johns Hopkins, and devoting all of his time to his ideas on a Thiel Fellowship.
back to top 

On June 24, UBI Index announced the Global Top 25 University Business Incubators of 2014. We would like to thank all participants of this year’s benchmark for great efforts in supporting entrepreneurs and helping the industry understand the importance of incubation.
back to top 

Valuations on venture-backed companies jumped again in the second quarter as the number of IPOs and the amount VCs invested in startups both hit post-dot-com highs, a new report from PitchBook Data shows.
The total amount invested has climbed steadily each quarter in the past year, jumping from $12.8 billion in Q2 of 2013 to $21.5 billion in the same period this year. The $13.9 billion raised in 76 new venture funds is also a recent high.
back to top 

A survey of healthcare and life science professionals and investors suggests healthcare M&A activity will surge past 2013 levels when there were 394 deals valued at $97 billion. The report by Bass Berry & Sims and Mergermarket indicates that market disruption, brought on by the Affordable Care Act, will lead to more consolidation deals across healthcare facilities, life science and healthcare IT companies.
The Affordable Care Act and HITECH Act are etched into the heart of most of these deals. That’s apparent from the facilities trying to figure out ways to cope with reduced Medicare reimbursements to the technology hospitals will need to adopt to fit in with change in payment models in the future. They also need to comply with electronic medical record requirements. Here are six trends that illustrate and factor in to the survey’s outlook.
back to top 

Educational experts have been telling us the future of employment opportunity will be in jobs requiring science, technology, engineering or mathematics training. At least in the D.C. area, the future is now.
A new study from the Brookings Institution finds the majority (55.1 percent) of job postings in the Washington area in the first quarter of 2013 required STEM skills. And not rudimentary skills either. More than 48 percent of all job postings required STEM skills and at least a bachelor’s degree.
back to top 

Anyone who wants a job next year at Anne Arundel Medical Center — whether as a surgeon or security guard — will have to prove they don’t smoke or use tobacco.
The Annapolis hospital’s new hiring policy might be controversial, but it is legal in Maryland and more than half of the United States. And it’s a type of job screening that is gaining favor with employers — from hospitals to companies such as Alaska Airlines — trying to control rising health costs and cultivate a healthier, more productive workforce.
back to top 

Reston-based New Atlantic Ventures joined a handful of other venture investors in backing Truveris, a New York startup whose cloud-based platform helps drive down the cost to companies of providing prescription drug benefits.
NAV Fund, an existing investor, joined New Leaf Venture Partners, Tribeca Venture Partners and First Round in Truveris’ $12.75 million Series C round, which was led by Canaan Partners.
back to top 

The heart is more forgiving than you may think — especially to adults who try to take charge of their health, a new Northwestern Medicine® study has found.
When adults in their 30s and 40s decide to drop unhealthy habits that are harmful to their heart and embrace healthy lifestyle changes, they can control and potentially even reverse the natural progression of coronary artery disease, scientists found.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

July 8
Planet Fitness

July 8
Johns Hopkins University Montgomery County Campus

July 17
Roof Bethesda

August 14
1776

August 14
Smokey Glen Farm Barbequers
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Scientific excellence is an integral part of any pharmaceutical company’s success. By paying attention to staff training and development at all levels, it is possible to embed vital life-long skills and behaviours into the scientists and professionals responsible for discovering and developing new medicines to treat patients.
MedImmune is the global biologics R&D arm of AstraZeneca and is committed to scientific innovation and medical progress. Our state-of-the-art facilities at Granta Park are home to over 550 members of the 2,500-strong global MedImmune team. To support its ambitions to help save lives and improve people’s health, MedImmune works hard to ensure that all employees, across all functions, are given the training they need to excel in their roles and work alongside their colleagues. MedImmune’s Cambridge site is therefore very pleased that it has recently been awarded Gold Standard Status for Training in Life Sciences by Cogent, the organisation responsible for skills in the life sciences sector and connected to government policies in this area.
back to top 

Emergent BioSolutions Inc. – Product Pipeline Review – 2014 is a new market research publication announced by Reportstack. This report provides an overview of the Emergent BioSolutions Inc.’s pharmaceutical research and development focus.
This report provides comprehensive information on the current therapeutic developmental pipeline of Emergent BioSolutions Inc.’s, complete with comparative analysis at various stages, therapeutics assessment by drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. It also reviews latest updates, and featured news and press releases, along with special features on late-stage and discontinued projects.
back to top 

GSK and Save the Children have announced the second annual $1 million Healthcare Innovation Award, which was established to identify and reward innovations in healthcare that have proven successful in reducing child deaths in developing countries.
Organizations from across the developing world can nominate examples of innovative healthcare approaches they have discovered or implemented. These approaches must have resulted in tangible improvements to under-5 child survival rates, be sustainable and have the potential to be scaled-up and replicated. Special attention will be given to work that aims to increase the quality of, or access to, healthcare for newborns.
back to top 

Johns Hopkins University is one of eight Baltimore universities and hospitals that today joined Mayor Stephanie Rawlings-Blake to sign a pledge to work together to grow and revitalize the city and to help solve some of its most pressing challenges.
The Baltimore City Anchor Plan targets four priority areas—public safety, local hiring, local purchasing, and quality of life in the city. In a memo introducing the plan, Rawlings-Blake said that the signing institutions, which are among the city’s largest employers, are integral to her goals of attracting new families to Baltimore and increasing jobs and investment.
back to top 

The BioMaryland Center is pairing up with a French life science organization to support a business venture between two companies.
BioMaryland and Medicen Paris Region will each invest $200,000 in a commercialization project between Opticul Diagnostics in Rockville and the French company Diafir. Each company will also put $200,000 toward the project for a total investment of $800,000.
back to top 

The University of Maryland School of Medicine’s Maryland Psychiatric Research Center (MPRC), a research center in the School’s Department of Psychiatry, was awarded a $10.7 million grant from the National Institutes of Health (NIH) to establish a Silvio O. Conte Neuroscience Research Center that will examine the causes of schizophrenia and search for possible new treatments. Schizophrenia is a devastating psychiatric disease, affecting one percent of people worldwide. Although its roots have been traced to abnormal early brain development, the cause remains a mystery, and current treatments are limited.
The five-year NIH grant*, one of only two awarded this year nationwide, will enable researchers to conduct breakthrough research, combining laboratory and clinical studies of a key chemical called kynurenic acid, a major breakdown product of the amino acid tryptophan. Kynurenic acid levels are increased in the brain of individuals with schizophrenia, and appear to contribute especially to the cognitive abnormalities, which are core symptoms of the disease and a major reason for the inability of people with the disorder to lead productive and fulfilling lives.
back to top 

The A. James Clark School of Engineering and the Maryland Technology Enterprise Institute (Mtech), an international leader in technology entrepreneurship education and venture creation, seeks a teaching faculty member to join its award-winning team.
Leveraging their experiences in technology entrepreneurship, the faculty member will teach approximately five courses per year, with an emphasis on undergraduates. Key topics may include customer discovery, marketing strategy, new product design and development, design for manufacturing, industrial design, concurrent engineering, and/or market development and commercialization.
The lecturer will be expected to use innovative teaching approaches to include collaborative learning, project based learning, flipped classroom, online learning, and civic engagement. This person will play an active role in collaborating with student entrepreneurs to launch new ventures.
back to top 

Whenever Baltimore-area companies sell themselves to out-of-state firms, economists and local leaders alike bemoan the loss. Another headquarters gone. Fewer corporate decision-makers here. Possible job cuts.
But Silicon Valley’s deals for two Columbia firms — the planned Micros Systems acquisition, announced last week, and Sourcefire last year — strike local entrepreneurs in an entirely different way.
back to top 

Maryland has a new strategy for growing biotechnology business: Roll out the welcome mat for international companies.
International bio companies looking to break into the American market must first get approval from the U.S. Food and Drug Administration. The FDA regulates medical devices and drugs. The approval process can be complicated even for American companies that are somewhat familiar with the agency.
back to top 

Today, Under Armour (NYSE:UA), the global leader in sports performance and innovation, announced plans for the 2014 Under Armour Future Show: The Connected Fitness Innovation Challenge, which asks innovators to submit concepts for the next generation of game-changing digital experiences, through apps and wearable technology, while utilizing the MapMyFitness software. Finalists will present their concepts to the Brand’s executive team in October, 2014 at Under Armour’s global headquarters in Baltimore, MD for a chance to win the Grand Prize of $50,000. Winners may then be eligible for an additional investment of up to $150,000. Innovators can now submit their ideas and innovations at idea.underarmour.com for consideration.
Entering its fourth year, Under Armour’s Future Show rewards the Brand’s innovation-inspired consumer base, incentivizing them to design new products that align with the Brand’s forward-thinking vision. The 2014 Future Show will identify the most creative minds in engineering and software development who can implement wearable, wireless and embedded apparel-based technology addressing one or more of the following needs: fitness assessment and training, readiness and recovery, sleep analysis or other novel usages that will help make athletes better.
back to top 

Cyber entrepreneur Mike Binko is launching a second Innovation Sandbox to give exposure to small startup companies.
The program will connect health care, medical and life science organizations with small vendors that are interested in working with the health care sector, said Binko, CEO of cloud services provider Kloudtrack.
back to top 

Kyushu University and the University System of Maryland exchanged memorandums of understanding on Tuesday to work together on cybersecurity education and research. It will be the first cooperation between a Japanese and U.S. university in the area of cybersecurity.
Setsuo Arikawa, the president of Kyushu University, visited Maryland to sign the memorandums with William Kirwan, the chancellor of the University System of Maryland, to create a partnership with the University of Maryland, Baltimore County.
back to top 

Johns Hopkins University affiliate Jhpiego has been awarded a five-year, $500 million contract to lead a federal global health program.
The Baltimore nonprofit organization will lead the Maternal and Child Survival Program, which targets preventable maternal and child deaths in 24 countries in Africa, Asia, the Caribbean and the Middle East that have the highest mother and child death rates. The award is from the U.S. Agency for International Development (USAID).
back to top 

ATCC, the premier global biological materials resource and standards organization, has licensed technology from the Centers for Disease Control and Prevention (CDC) and partnered with Thermo Fisher Scientific to bring a rapid and cost-effective PCR-based method of monitoring HIV drug resistance to resource-limited countries. The ATCC® HIV-1 Drug Resistance Genotyping Kit was optimized for off-the-shelf detection, sequencing, and genotyping of HIV-1 genomic mutations more commonly observed in resource-limited countries, and was attuned to sample collection methods most often employed in developing countries, including dried blood spots. Together, with ongoing CDC programs to train scientists in geographically dispersed regions, such as Sub-Saharan Africa, Central America, and Southeast Asia, this collaboration serves to advance applied public health initiatives to understand the growing problem of HIV drug resistance and, ultimately, improve patient outcomes.
One of the most difficult aspects of supporting resource-limited countries with temperature-sensitive reagents is being able to supply materials under stable conditions. This challenge was addressed by ATCC’s long-standing history and expertise in global cold-chain distribution of biological materials routinely used in research to further advances in human health. “Researchers around the globe rely on ATCC as the leader in the production and distribution of reagents for diseases that impact the world, such as influenza, tuberculosis, and malaria,” said Dr. Ted Mullins, Program Manager for ATCC Biological Services. “The release of these kits to World Health Organization designated and CDC-supported PEPFAR (President’s Emergency Plan for AIDS Relief) genotyping labs for the surveillance of drug resistance in HIV patients demonstrates yet another facet of our commitment to improving global health.”
back to top 

A few years ago, the Maryland legislature appointed a panel to assess the way it was funding higher education. As part of its scope, the panel evaluated the funding needs of the state’s historically black colleges and universities (HBCUs), paying particular attention to the research infrastructure needs at Morgan State University, which in 2005 had received the coveted Carnegie designation of “Doctoral Research University” without any additional infusion of state resources. It achieved this designation because it annually awarded the requisite number of doctoral degrees and received sufficient external federal research funding to qualify.
The state’s study found that unlike the University System of Maryland research campuses in College Park and Baltimore city and county, Morgan lacked most of the infrastructure components typically found at campuses with a research university label. Morgan’s faculty has much higher than average teaching loads, and its research laboratory space and equipment were inadequate. The study concluded that an investment in Morgan’s research platform would be required to allow it to adequately compete with research campuses with better developed infrastructures. But while state investment in capital facilities at Morgan has improved, its investment in enhancing Morgan’s research mission has not.
back to top 

Potential medical breakthroughs now being developed in Maryland, the fourth largest biopharma cluster in the U.S., will be the focus of the BioMaryland booth at BIO International 2014 at the San Diego Convention Center this week. The innovations range from a surgical tool that can speed and enable heart operations for patients who currently are not candidates for traditional open heart surgery to a simple diagnostic test to reduce the spread of malaria.
The following companies will be presenting their work and research at BIO International on Tuesday:
back to top 

Niall O’Donnell fancies himself an archaeologist of the pharmaceutical persuasion. His firm, RiverVest Ventures, is scoping out the failed and forgotten drugs of big pharma, building companies to repurpose these benched meds for new indications.
The aim is to find drugs that have passed for safety in clinical trials, but may have shown limited efficacy in the initial disease they aimed to treat.
back to top 

After stepping up their R&D spending last year, biotechnology companies worldwide must deploy that capital to create more value from their research, EY concluded in this year’s 28th annual edition of its industry report, released Tuesday at the Biotechnology Industry Organization (BIO)’s 2014 International Convention in San Diego.
Beyond Borders: Unlocking Value focused on three strategies for creating more value—“Adaptive” clinical trials that allow biotechs to tweak their hypotheses and shift R&D spending based on clinical data; “Precision medicine” that identifies patient subgroups most likely to benefit from a new therapy; and cross-industry collaborations during precompetitive phases, spearheaded by big pharma.
back to top 

May 18 was a glorious Sunday in the Boston area. Tens of thousands of families and friends descended on one of the nation’s best-known education corridors to celebrate the class of 2014. My husband and I were among the happy crowds joined by my family from Shanghai for our son Andrew’s graduation from Tufts University.
We fully immersed ourselves in the joy of gathering, cheering, listening, reflecting and photo snapping—including some selfies. Our weekend of celebration culminated with Andrew’s commencement speech on behalf of the Sociology Department. In a mother’s unbiased opinion, his 5-minute speech was brilliant, perceptive, and entertaining, an affirmation of the value of his college education in shaping his outlook on self and the society. Surrounded by family, I cheered and laughed as I listened to him. It was a moment of pure joy and pride that no other accomplishments of my own could possibly match.
back to top 

BIO BizLink is an online platform that connects life sciences companies with an unparalleled community of pre-clinical and clinical R&D service vendors
BIO BizLink is a portal to an unparalleled community of R&D vendors – including pre-clinical and clinical Contract Research Organizations (CROs), Contract Manufacturing Organizations (CMOs), and regulatory consultants – and a platform to take the administrative hassle out of RFP, vendor, and project management.
User on the platform can explore the community to find specific services and expertise; review company profiles and key personnel; start conversations about prior experiences; invite vendors to submit proposals; and execute confidentiality agreements. BIO BizLink was developed and launched as a cooperative effort among BIO, its State and Regional Affiliates and OnDeckBiotech.
back to top 

Healthbox, a leading business accelerator focused on healthcare technology and technology-enabled companies, today confirmed it is taking its accelerator program to Salt Lake City this August. Through partnerships with innovative organizations Intermountain Healthcare and HealthEquity, in addition to Zion’s Bank and BD (Becton, Dickinson and Company), Healthbox is sourcing companies, regardless of development stage, that meet the needs of a rapidly changing healthcare industry. The companies selected for the 16-week program will have unique access to Healthbox strategic partners, gaining an understanding of the key challenges plaguing these organizations and how to adapt their solutions to truly solve these challenges.
“We are thrilled to expand to Salt Lake City and build relationships with the local community. Utah has one of the fastest growing economies, and we believe there is a large opportunity to elevate the recognition of the area’s talented healthcare entrepreneurs,” says Nina Nashif, founder and CEO of Healthbox. “As Healthbox continues to grow, it is important that we commit to communities with both strong local health economies and an interest in advancing innovation.”
back to top 

Reston-based New Atlantic Ventures joined a handful of other venture investors in backing Truveris, a New York startup whose cloud-based platform helps drive down the cost to companies of providing prescription drug benefits.
NAV Fund, an existing investor, joined New Leaf Venture Partners, Tribeca Venture Partners and First Round in Truveris’ $12.75 million Series C round, which was led by Canaan Partners.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

July 8
Johns Hopkins University Montgomery County Campus

September 15-16
Sheraton Pentagon City

September 15
Various Locations
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Richard Bendis and BioHealth Innovation were among the small businesses and individuals honored by the US Small Business Administration at the 2014 Tibbetts awards. Winners of the award have been critical in supporting the SBIR/STTR program in many different ways and are at the forefront of driving innovation. Read the full PR Newswire press release here.
The BHI SBIR/STTR Federal Funding Assistance Program offers biohealth companies support in preparing applications for federal funding inclusive of SBIRs, STTRs, and other federal government awards. Companies submit their federal funding concepts and receive pre-proposal feedback to help troubleshoot and strengthen your application. Further support from professional consultants and service providers is available to assist in improving your application.
For more information on the program, contact Ethan Byler.
back to top 

Governor Martin O’Malley and The Maryland Department of Business and Economic Development announced today that Cellphire, a Rockville biotechnology company, has received a $1 million investment through the State’s InvestMaryland program. Cellphire is developing stabilized cellular products, including freeze-dried platelets that can be stored for years, for use in a range of advanced therapeutic and diagnostic applications.
“Supporting entrepreneurs and innovators like those at Cellphire is a central piece of Maryland’s broad support of the startups and small businesses that move our Innovation Economy forward and keep our State competitive in the 21st Century,” said Governor O’Malley. “Maryland is a center of healing and discovery. The technology being developed by Cellphire has the potential to improve care and save lives around the globe.”
back to top 

Cambridge UK medical technology heavyweights are in the front line of a battle to speed diagnosis and potential treatment of dementias.
MedImmune, IXICO, Johnson & Johnson Innovation (now with a Babraham base) and the University of Cambridge are in a consortium created by The Medical Research Council in the form of the UK Dementias Research Platform (UKDP) – a £16 million public-private partnership set up to speed up research into dementias.
back to top 

Qiagen, a Netherlands based holdings company announced that its artus CMV RGQ MDx Kit for human cytomegalovirus (CMV) has been approved by the US Food and Drug Administration (US FDA) under a full premarket approval (PMA). The test is the only FDA-approved PCR-based assay optimised for low- to mid-throughput testing of CMV. With a turn-around time of approximately three hours, the assay provides faster results than other PMA approved tests. artus CMV RGQ MDx runs on Qiagen’s Rotor-Gene Q MDx real-time PCR platform, which was cleared by the FDA in 2012 and is a member of the QIAsymphony modular family of automated instruments.
“We are very pleased to add yet another FDA approved diagnostic kit to our menu of FDA approved or cleared content for a core element of our flagship QIAsymphony modular family of automated instruments. This flexible platform is driving the dissemination of molecular diagnostics by delivering efficient, reliable workflows in low- to mid-throughput settings, which represent the largest market opportunity in terms of placements,” said Peer M. Schatz, chief executive officer of Qiagen. “Our artus CMV assay is the fastest test approved for quantifying CMV viral loads in organ transplant patients. In addition to helping save lives with its clinically proven usefulness, the FDA-approved artus test creates economic value by reducing the time and money many labs and hospitals currently must spend validating lab-developed CMV tests and analyte-specific reagents. More than one million CMV tests are performed on US transplant patients each year and we believe the artus CMV kit will provide significant value for laboratories, patients and the healthcare system.”
back to top 

When Mike Oberst walks through the doors of MedImmune in Gaithersburg each day, he resumes his important work on developing a newer, promising type of cancer treatment.
The scientist has focused his attention on immunotherapy as a way to eliminate cancer cells in patients. The treatment aims to harness the power of one’s immune system to fight cancer.
back to top 

Tuesday, July 8, 2014 6:00 PM – Johns Hopkins University Montgomery County Campus, Rockville, MD
Guest Speakers:
GAUTAM GULATI, MD, MBA, MPH
Dr. Gulati is the Chief Medical Officer and Head of Product Innovation for Physicians Interactive Holdings, where he leads a world-class team to ideate, design, build, and deploy disruptive solutions for a health audience. In addition to his executive role, he serves as an Adjunct Professor of “Medical Innovation and Entrepreneurship” at Johns Hopkins University Carey Business School, sits on numerous company boards, and speaks at a variety of events around the world.
Over his 20+ year career, Dr. Gulati has been combining his diverse experiences, creative juices, and passionate voice to help take on a bigger challenge…to treat our troubled health system. He has earned an impeccable reputation for his ability to transform organizations – both big and small – to meet the future innovative demands of the health industry. Along this journey, he has been dubbed both a “health hooligan” and “physician artist”, encapsulating the creative characteristics that allow him to meld his various vantage points as a clinician, executive, designer, professor, advisor, entrepreneur, speaker, and technology advocate.
PAUL FEARIS, CEO Clinvue
Mr. Fearis obtained Masters Degrees in both mechanical engineering and industrial design and as a consequence has been involved in product development throughout his career. An entrepreneur at heart, with his partners, Mr. Fearis formed Clinvue, a company which marries his passion for innovation with his love of understanding people and solving worthwhile problems.
Peter Davis, JD
Mr. Davis is a partner in Whiteford, Taylor & Preston’s Technology and Intellectual Property Practice, located in Baltimore, Maryland. He is a former U.S. Patent Examiner, and for the last 23 years he has balanced his time between patent prosecution, patent litigation, and strategic patent counseling for many of the world’s best-known companies. He has worked in virtually all areas of technology, including biotechnology, pharmaceuticals, medical devices, electronic devices, software, light and heavy machinery, and automotive. He can speak the languages of both the inventor and the layman, translating inventions into terms that patent examiners, judges and juries find compelling. Mr. Davis received his bachelor’s degree from Cornell University (1987) and his JD from Catholic University (1993).
back to top 

The FLC invites you to submit images of your lab’s work for its 2015 planner! Why keep all of that hard work to yourself? Share it with the more than 10,000 planner recipients throughout the FLC community, including members of Congress, scientists, tech transfer professionals, and members of academia and industry. The FLC planner features an array of images displaying the innovative research and development that occur daily in our nation’s federal labs.
To submit images of your lab’s work, carefully read the submission criteria below. You also may want to coordinate your submission with, or through, your agency’s FLC representative or public affairs office.
back to top 

Over the last two and a half years the National Science Foundation I-Corps has taught over 300 teams of scientists how to commercialize their technology and how to fail less, increasing their odds for commercial success.
After seeing the process work so well for scientists and engineers in the NSF, we hypothesized that we could increase productivity and stave the capital flight by helping Life Sciences startups build their companies more efficiently.
back to top 

A collaboration between the National Science Foundation (NSF) and the National Institutes of Health will give NIH-funded researchers training to help them evaluate their scientific discoveries for commercial potential, with the aim of accelerating biomedical innovations into applied health technologies.
I-Corps at NIH is a pilot of the NSF Innovation Corps (I-Corps) program specially tailored for biomedical research. Academic researchers and entrepreneurs with Small Business Innovation Research and Small Business Technology Transfer (SBIR/STTR) Phase I awards – awards that establish feasibility of proof of concept for commercializable technology – from participating NIH institutes will be eligible to apply to I-Corps at NIH. NIH will begin outreach to the small business research community with a June 25 program briefing at the 2014 BIO International Convention in San Diego, and a webinar on July 2.
back to top 

We are delighted to announce a new collaboration between the National Institutes of Health (NIH) and the National Science Foundation (NSF) to empower entrepreneurial scientists and advance the Lab-to-Market priorities set forth in the President’s Management Agenda. The Federal government invests over $130 billion on research and development (R&D) each year, and the President’s 2015 budget supports a sustained commitment to accelerate the transfer of promising Federally-funded technologies from the laboratory to the commercial marketplace.
Some academic researchers and entrepreneurs who receive SBIR or STTR funding from NIH will now be eligible to participate in a pilot of the NSF Innovation Corps (I-Corps™) program that is specially tailored for biomedical technologies. First launched in 2011, the NSF I-Corps program is based on the “Lean Launchpad” curriculum developed by entrepreneurship expert Steve Blank to improve how tech start-ups bring their products into the marketplace. This intensive, mentor-driven experience is changing the way that NSF-funded researchers think about the commercialization process, and now it will be available for NIH-funded researchers as well.
back to top 

The U.S. Food and Drug Administration on Tuesday issued proposed guidelines for the pharmaceutical and medical device industries for posting information on social media networks and correcting misinformation posted by others.
The long-awaited guidance would effectively limit the amount of product advertising a company can do on sites where character space is limited, such as Twitter.
back to top 

At Novartis’s research lab in Cambridge, Massachusetts, a large incubator-like piece of equipment is helping give birth to a new era of psychiatric drug discovery. Inside it, bathed in soft light, lab plates hold living human stem cells; robotic arms systematically squirt nurturing compounds into the plates. Thanks to a series of techniques perfected over the last few years in labs around the world, such stem cells—capable of developing into specialized cell types—can now be created from skin cells. When stem cells derived from people with, say, autism or schizophrenia are grown inside the incubator, Novartis researchers can nudge them to develop into functioning brain cells by precisely varying the chemicals in the cell cultures.
back to top 

Medtronic Inc., an active acquirer and financial backer of new medical technologies, has moved its tax domicile overseas with the $42.9 billion acquisition of rival Covidien PLC, a deal that Medtronic says will free up billions of dollars that can be more easily and flexibly deployed to technologies being developed in the U.S.
Medtronic headquarters in Minneapolis Bloomberg News But investors in these U.S.-based technologies see things differently. The merger means that the medical-technology industry is consolidating, several venture capitalists said, which takes options off the table for young companies struggling to bring new treatments onto the market.
back to top 

The ETC (Emerging Technology Centers: http://www.etcbaltimore.com) — Baltimore City’s award-winning technology innovation center–welcomed last week a European Delegation of entrepreneurs, policy makers and Members of Parliament who were invited to the United States under the auspices of the Department of State’s International Visitor Leadership Program and coordinated by the World Trade Center Institute (WTCI) in Baltimore, Maryland. Two weeks ago, the ETC hosted another European Delegation led by Dr. Rob de Wijk, Director of The Hague Security Delta.
“At ETC, we have always been pioneers,” said Deb Tillett, ETC’s President. “We work hard every day to innovate and move forward. We are so pleased that these distinguished delegations chose to visit us to see how we do what we do in Baltimore and how we stay on the leading edge of technology and best practices to help our emerging companies grow and become successful, all business is global these days and our companies are always looking for the next customer and opportunity,” she added.
back to top 

Top pharmaceuticals face a dilemma in that many blockbuster drugs are losing patent protection and existing pipelines may not compensate for lost revenues. The choice for many is layoffs, office closings, downsizing or to innovate with new products. Most companies will do the former if they must but prefer the latter. Rather than the megamergers that achieve big cost savings through layoffs and factory closings, most drug companies are aiming for transactions that grow their bottom line. In the last few years, this trend has resulted in a complex series of deals and transactions, ranging from complete buyouts to licensing transactions to a variety of collaborative arrangements.
back to top 

The Nasdaq Biotechnology Index (NBI) has been on a wild ride in 2014 with a 20 percent gain in the first two months, a 24 percent drop from late February to mid-April, and a rebound of 16 percent off April lows by early June. This recent sell-off follows a 130 percent gain over the last three years and the debut of 100 biotech IPOs, leading some investors to ask if the recent sell-off is the start of a bubble bursting.
Indeed, the biotech sector has been hot for the last couple of years, but there is very little evidence to support that biotech has been forming a bubble ready to pop.
back to top 

Just how valuable is “technology transfer” for universities? This question is addressed in “More than Money: The Exponential Impact of Academic Technology Transfer,” an article from the National Academy of Inventors (NAI) that examines the impact of landmark 1980 legislation that facilitated technology transfer from the academic inventors’ “bench” to commercialization and the far-reaching and beneficial changes for universities and communities that have resulted.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 23-26
San Diego, CA

June 26
University of Maryland BioPark

July 8
Johns Hopkins University Montgomery County Campus

September 15-16
Sheraton Pentagon City

September 15
Various Locations
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 BHI is pleased to announce the promotion of Amanda Wilson to the position of Director, Finance & Human Resources. Amanda joined BioHealth Innovation, Inc. two years ago as the Operations Manager. BHI is pleased to announce the promotion of Amanda Wilson to the position of Director, Finance & Human Resources. Amanda joined BioHealth Innovation, Inc. two years ago as the Operations Manager.
Over the past two years, Amanda has transitioned BHI’s accounting method from cash to accrual. She has ensured BHI compliance with Generally Accepted Accounting Principles (GAAP), and has represented BHI through two successful audits. In addition, Amanda has developed human resources policies and procedures and has maintained employee access to BHI’s benefits package. Amanda recently completed her MBA at The George Washington University, and is able to augment her pre-existing skills through this degree.
Join us in congratulating Amanda on a well-deserverd promotion!
back to top 

Rockville-based CytImmune received $100,000 from Montgomery County’s equity investment program to help the company advance its industry-leading work in the development of tumor targeted nanomedicines.
The County’s investment was made by the Montgomery County Department of Economic Development, in conjunction with a $200,000 equity investment made by the Maryland Venture Fund, which is administered by the Maryland Department of Business and Economic Development (DBED). Both the County’s and State’s investments in CytImmune are part of a broader strategy to support the growth and development of innovative companies.
back to top 
 The Chinese Biopharmaceutical Association, USA (CBA) will host the 19th Annual Conference at the University of Maryland Shady Grove Conference Center on Saturday, June 21st, 2013. The theme of this year’s conference is “Advancement and Global Opportunities in Innovative Biopharmaceutical Development”. The Chinese Biopharmaceutical Association, USA (CBA) will host the 19th Annual Conference at the University of Maryland Shady Grove Conference Center on Saturday, June 21st, 2013. The theme of this year’s conference is “Advancement and Global Opportunities in Innovative Biopharmaceutical Development”.
Six conference sessions:
- Advancement in Drug Discovery of Biopharmaceuticals
- Novel Therapeutics and Vaccine Product Development
- Bridging US-China Partnership for Biopharmaceuticals
- Biopharmaceutical Manufacturing and Regulation Compliances
- SCBA Joint Session: A Kaleidoscopic View of Cancers
- Legal Challenges in Trade Secret Protection
The conference provides a perfect venue for the attendees to meet and network with high-caliber speakers, high-level industry leaders and high-profile governmental officials from both China and the U.S., to explore potential partnership and collaboration opportunities.
Flyer
back to top 

When GlycoMimetics Inc. announced a license agreement with Pfizer three years ago, the decision seemed like a no-brainer. This deal, which surrounded the sickle cell drug candidate GMI-1070, was worth up to $340 million, after all. The Gaithersburg company seemed to be in an enviable spot, while some of its Maryland counterparts struggled.
Behind the scenes, the decision to partner with big pharma didn’t come easy. CEO Rachel King, speaking this morning at a Tech Council of Maryland panel in Bethesda, recounted the calculus behind the move.
back to top 
September 2014 is bringing 5 NEW CLASSES to our schedule that will help you
BE THE ENTREPRENEUR THAT YOU WANT TO BE!

Aspiring Entrepreneurs:
1. The Intentional Entrepreneur: This one day class will help you identify your entrepreneurial skills and abilities, as well as address any concerns you may have about owning your own business. This class is a must for anyone considering starting their own business!
2. The Intentional Entrepreneur for Veterans: For veterans thinking about starting a business.
Early Stage Entrepreneurs:
3. New Venture: For the entrepreneurs in the early stages of business development.
4. New Venture for Veterans: For veterans seeking to launch an idea.
5. Tech Venture: For entrepreneurs in the tech or life sciences field seeking to start a business. (BHI EIR Todd Chappell will be one of the instructors teaching this class)
back to top 

Novavax Inc. (Nasdaq: NVAX) said Wednesday it has raised $115 million in gross proceeds by selling 28.75 million shares of its common stock, which the Gaithersburg biotech plans to pour into its pipeline.
Some of the net proceeds will go toward advancing Novavax’s Respiratory Syncytial Virus (RSV) nanoparticle vaccine into a Phase 2 trial in elderly subjects this year. The biotech is also studying its RSV vaccine for pediatric and maternal populations.
back to top 

EntreMed (Nasdaq: EMND) has been through plenty of turbulence. In 1998, it was the subject of a breathless front page New York Times story suggesting the biotech was on the verge of curing cancer, based on promising mice studies. Those hopes (and EntreMed’s share price) deflated in the coming years, when the company found itself shedding jobs and executives and struggling to survive.
But through it all, EntreMed remained EntreMed. No longer: the Rockville company’s shareholders have voted to change its name to CASI Pharmaceuticals, as of June 16. The company will now trade under the ticker symbol CASI.
back to top 

MedImmune, its parent company AstraZeneca and a handful of other pharma companies and health groups announced a broad lung cancer trial on Monday, looking to use patients’ genomic profiles to match them with therapies.
The Lung Cancer Master Protocol, or Lung-MAP, trial will explore five product candidates as treatments for advanced squamous cell lung cancer. That includes MedImmune’s MEDI4736, according to spokeswoman Tracy Rossin.
back to top 

Maryland has retained top rankings in the U.S. Chamber of Commerce Foundation’s annual Enterprising States study released Wednesday.
For the third consecutive year, the State ranked No. 1 in Innovation and Entrepreneurship. For the second year in a row, Maryland ranked third in the nation for its Talent Pipeline.
back to top 

Several Baltimore startups are among the winners of this year’s Maryland Incubator Company of the Year awards.
The winners were announced Tuesday evening at an awards ceremony at the American Art Visionary Museum in Baltimore. The awards are supported by the Maryland Technology Development Corp., the Maryland Department of Business and Economic Development, the Maryland Business Incubation Association, M&T Bank and several other state companies.
back to top 

The University of Maryland, along with the George Washington University and Virginia Tech, have added the Johns Hopkins University to the National Science Foundation’s Innovation Corps (I-Corps ™) regional collaboration called DC I-Corps. JHU becomes the newest member university of the National Science Foundation’s National Innovation Network.
The NSF has approved a request from the three original universities to officially include Johns Hopkins in the I-Corps program’s “node” in the Mid-Atlantic called DC I-Corps, which was formed last year with $3.75 million in NSF funding. It is one of five regional nodes established nationwide by the NSF, and the first to expand its membership. Together, these five nodes currently form the basis of the National Innovation Network, which links together select universities with established entrepreneurs and venture capitalists to train faculty and student researchers from throughout the U.S. to transform ideas into products and get them on the market.
back to top 

Two early career physician-scientists, Peter de Blank and Matthew R. Steensma, have been named inaugural winners of the Francis S. Collins Scholars Program in Neurofibromatosis Clinical and Translational Research, sponsored by the Neurofibromatosis Therapeutic Acceleration Program (NTAP) at The Johns Hopkins University. The program will create a community of expert clinician-scientists and groom them to be leaders in neurofibromatosis type 1 (NF1) research and clinical care. The awards will be presented at a ceremony on Tuesday, June 10, at the Whittemore House in Washington, D.C.
“It has become increasingly hard for young clinician-scientists to get the funding and dedicated time necessary to become leaders in translational science for rare diseases such as NF1,” says Jaishri Blakeley, M.D., director of the Johns Hopkins Comprehensive Neurofibromatosis Center and NTAP. “We created the Francis S. Collins Scholars Program, recognizing that a cadre of well-trained and dedicated clinician-scientists focused on NF1 is critical in order to make the scientific leaps that are possible in this modern era.”
back to top 

Researchers at WellDoc® today presented updated results of its hypoglycemia prediction technology at this week’s 2014 American Diabetes Association 74th Scientific Sessions. In previous work, using just one week of blood glucose data, WellDoc’s models were shown to predict correctly 90 percent of the time that hypoglycemia would occur the following day. Today, WellDoc announced an enhanced model that incorporates both glucose and medication data has demonstrated the ability to predict hypoglycemia within a specific hour.
“The challenge in predicting hypoglycemia is that most patients with type 2 diabetes measure their blood glucose once or twice per day. This so-called ‘sparse data’ makes mathematical forecasting difficult,” states WellDoc Chief Data Science Officer, Anand Iyer, Ph.D. “We used machine learning algorithms which allow the computer to detect patterns and make predictions after being trained on thousands of data points.”
back to top 

Upcoming Funding Opportunity Deadlines -NEW FORMS-C Required for SBIR/STTR applications
The next NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Omnibus deadline is only two months away on August 5, 2014, which means your small business should be preparing your application.
back to top 

kloudtrack®, a leader in cybersecurity and cloud computing (cyber|cloud) technologies and solution services for sensitive data, process management and Governance, Risk and Compliance (GRC), will team up with the University of Maryland’sRobert H. Smith School of Business and Cisco Systems (NASDAQ:CSCO) to establish the first Innovation Sandbox™ exchange catering to the innovation, workforce development and technology roadmapping needs of public and private sector healthcare, and medical and life science (HealthTech) organizations.
The project was announced as part of today’s Maryland Economic Development Association Summer Conference at UMD, themed “Health Innovations: Impact on Economic and Workforce Development.”
back to top 

EY unveiled on Thursday evening its 2014 Entrepreneur of the Year Maryland winners in eight categories.
The honorees were announced in front of a packed crowd at a black-tie event at the Hilton Baltimore. The awards program recognizes high-growth entrepreneurs who demonstrate excellence and success in such areas as innovation, financial performance and personal commitment to their businesses and communities.
back to top 

America is still the world leader in creating new medical technologies that have the potential to save lives. So why is it so tough for the entrepreneurs and investors behind these new products to make a living here?
One reason, according to venture capitalists, is long, costly delays before getting new treatments to market, in some cases caused by health insurers dragging their feet when it comes to agreeing to reimburse doctors for using new medical products.
back to top 

Maryland is missing out on as many as 7,000 jobs and millions of dollars of investment as companies build data centers in neighboring Virginia, according to a report from Baltimore accounting firm Glass Jacobson.
Data centers provide cloud storage and are crucial to supporting the kinds of technology firms that have been opening in and relocating to Baltimore.
back to top 

After all the angst generated by the Affordable Care Act, and all the punditry, noise, and debate that accompanied its rollout, you might conclude that there are no practical solutions to our healthcare challenges. But, of course, there are new answers and solutions and new, creative approaches to solving healthcare problems. You just have to look at innovative, private sector enterprises. And if you do, you might find one of the more innovative answers to eldercare right there in your old-fashioned television set. Just ask Kian Saneii, CEO of Independa.
But first, a little background. One of the more troubling and difficult areas of healthcare is the growing needs of the elderly, including both preventive care and the management of long-term, chronic illnesses. This has been a well-understood fact for quite some time. Saneii and the Independa team he began assembling in 2009 set out to find practical real-world solutions to these challenges. How they did this is an instructive case study in innovation, pragmatism and common sense.
back to top 

A new report from Rock Health looking at the future of the biosensor wearables market shows a market in transition. The next generation of wearables is more targeted towards patient populations, particularly chronic conditions. In a Google hangout about the report, Malay Gandhi, a co-author of the report, talked about some of the qualities that are making these wearables more appealing to consumers and the b2b market and features that will give them staying power.
Athletic trackers aimed at the mass market have lost ground Nike’s exit from the wearables market shows there are far more fitness tracking devices than the market can support. There’s also a certain amount of consumer fatigue because the accuracy of fitness bands can vary. It’s difficult to keep most consumers interested in using them after six months. That prompted The New York Times article comparing these wristbands to “digital snake oil.”
back to top 

This brief analyzes entrepreneurship and job creation in the U.S. life sciences sector—defined as the group of industries engaged in the application of biological science and related knowledge for commercial purposes, primarily for human health care. This definition contains three major subgroups: drugs and pharmaceuticals; medical devices and equipment; and research, testing, and medical laboratories.
Building on previous research that highlights the importance of entrepreneurship and business dynamism to innovation, productivity, and net job creation, this brief analyzes how those trends apply to the life sciences sector. Overall, the life sciences sector plays an outsized role in new job creation and makes important contributions to entrepreneurship—not to mention the perhaps immeasurable benefits these firms play in enhancing and extending human life.
back to top 

Tiny biotech startups will have a new place to germinate in the San Francisco Bay Area. Healthcare giant Johnson & Johnson’s Janssen Labs division is opening a new 30,000 square foot incubator in the biotech-rich suburb of South San Francisco.
The flexible space, complete with common rooms, wet labs, and offices, could hold as many as 50 companies, according to Melinda Richter, a Bay Area incubator veteran tapped last year to head Janssen Labs nationwide. That would roughly double the group’s nationwide capacity, part of the international company’s aggressive reach beyond its walls to find, and fund, new science and technology. “We’re taking a big footprint,” Richter said, with half devoted to shared research equipment and services and half to space that can be customized to individual tenants.
back to top 

The heightened private equity and venture capital (PEVC) deal activity in the global healthcare industry during the recession years, 2008-2010, witnessed a decline post-2010. However, the fall in deals was not uniform among the constituent sectors, with the pharmaceutical, biotechnology and healthcare equipment sectors experiencing a much sharper decline in investor interest than the healthcare technology and provider segments. Investors started to bet on providers based with the conviction they can provide quicker and safer returns than the pharmaceutical and biotechnology space, which is ridden with regulatory challenges and patent expiries.
New analysis from Frost & Sullivan’s Private Equity and Venture Capital Investment in the Global Pharmaceutical and Biotechnology Industry reveals the total number of PEVC deals in the pharmaceutical and biotechnology industry decreased from 1063 in 2010 to 480 in 2013. Though the returns from the pharmaceutical and biotechnology industry have been dwindling, they are better compared to the performance of other industries.
back to top 

Although it seems raising as much venture capital as humanly possible is Silicon Valley’s mantra, there are reasons to be cautious when signing termsheets. Startups raising a couple of million dollars had a median exit price of more than $10 million, while the outcome for those raising double that was actually worse, a recent study shows.
The report, by San Francisco-based Exitround, a marketplace for M&A deals for small tech companies, found companies raising $2 million to $3 million were more likely to exit at a valuation over $10 million, while for startups raising $3 million to $10 million the median exit price was less.
back to top 

A U.S. Senate panel on Tuesday approved a budget bill that would increase funding for the National Institutes of Health by $605 million for the fiscal year that begins October 1.
Lawmakers on the Senate’s appropriations subcommittee that oversees education, health and labor programs passed legislation that would increase the NIH’s budget to nearly $30.5 billion in the coming year. That $605 million jump represents a greater increase than the $198-million increase the Obama administration had requested.
back to top 
A highly personalized medical technique is allowing patients with advanced kidney cancer to live nearly three times as long as they normally do. In an experiment involving 21 patients, around half lived more than two and half years after diagnosis with kidney cancer that had begun to spread. Five patients are alive after more than five years.
“That seems to be out of proportion with what you would expect for any commercial therapy and longer than what you would expect from patients with similar prognostic variables,” says Robert Figlin, an oncologist at Cedars-Sinai Samuel Oschin Comprehensive Cancer Institute in Los Angeles, who is leading the study.
back to top 
I read an interesting Wall Street Journal article recently that discussed the war between tech companies to control future consumer distribution platforms. The article explains that the cash-flush giants “…each want to own the digital platform where people communicate, shop and seek entertainment.” This got me thinking about platforms in healthcare. read an interesting Wall Street Journal article recently that discussed the war between tech companies to control future consumer distribution platforms. The article explains that the cash-flush giants “…each want to own the digital platform where people communicate, shop and seek entertainment.” This got me thinking about platforms in healthcare.
Right now, it’s fair to say that there are three major healthcare platforms. Insurance companies such as Aetna, WellPoint, United Healthcare and Humana have substantial members. However, mentioned in the article is the continual need for innovation. Tech behemoths are“…aware of all the big companies that died because they rested on their laurels.”
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 15-19
Washington, DC

June 16-18
Gaylord Hotel and Conference Center at Washington DC National Harbor

June 15-19
Washington, DC

June 19
Germantown Innovation Center

June 21
University of Maryland Shady Grove Conference Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
– BHI offers program that guides biohealth startups in securing federal funding –
ROCKVILLE AND BALTIMORE, MARYLAND, June 10, 2014 – BioHealth Innovation, Inc. (BHI) announced today that two local biohealth companies to which it has provided strategic assistance, N5 Sensors, Inc. and BioDatomics, have secured Small Business Innovation Research (SBIR) awards. N5 Sensors, a BHI client, received two awards: one from the Enivronmental Protection Agency (EPA) and one from the National Science Foundation (NSF). BioDatomics, which participated in a federal funding assistance program offered by BHI, secured an SBIR grant from the National Institutes of Health (NIH)1. The U.S. federal government’s SBIR program encourages domestic small businesses to engage in federal research and development that has the potential for commercialization. The SBIR program empowers these companies to develop their trade and provides a path to profitability.
“BHI is dedicated to supporting start-up biohealth companies in Central Maryland in their submission of competitive SBIR applications,” said Ethan Byler, Director, Innovation Programs, BioHealth Innovation, Inc. “We congratulate N5 Sensors and BioDatomics on receiving SBIR grants from the EPA, NSF and the NIH, respectively.”
back to top 

Senseonics, a privately held medical device company focused on the development and commercialization of the first fully implantable, long-term continuous glucose monitoring (CGM) system, announced that it has raised an additional $20 million of private equity financing. Senseonics also announced that Mirasol Panlilio, formerly of Abbott Diabetes Care and LifeScan, has joined the company as Vice President of Global Sales and Marketing.
Current investors Anthem Capital, Delphi Ventures, Greenspring Associates, Healthcare Ventures, New Enterprise Associates and other strategic partners all participated in the financing round. Senseonics intends to use the proceeds to continue its product development initiatives including completing pivotal trials in Europe, obtaining CE mark, and initiating IDE trials in the United States. “We’re very happy of the continued support from our investors as we near the completion of the product development efforts for our first generation long-term CGM system, “ said Tim Goodnow, CEO and President. Senseonics has recently begun its European pivotal trials and expect to complete site initiation of all seven European sites before the end of summer.
back to top 

Rockville-based CytImmune Sciences Inc. has raised $400,000 from the Maryland Venture Fund, Montgomery County and an unnamed venture investor to push Aurimune — a drug that pairs a decades-old tumor-fighting agent with gold nanoparticles — into phase 2 trials.
The Maryland Venture Fund led the round with $200,000, with another $100,000 coming from the Montgomery County Department of Economic Development and the unidentified investor.
back to top 

Ventana Medical Systems, Inc. (Ventana), a member of the Roche Group and MedImmune, the global biologics research and development arm of AstraZeneca, today announced they are jointly developing a PD-L1 (SP263) immunohistochemistry assay to enroll patients in clinical trials for MedImmune’s MEDI4736 anti-PD-L1 therapy for non-small cell lung carcinoma. This includes the recently commenced MEDI4736 ATLANTIC trial that will enroll only patients who express PD-L1 as determined by the VENTANA assay.
MEDI4736 is an investigational, engineered, human monoclonal antibody directed against programmed cell death ligand 1 (PD-L1). Signals from PD-L1 help tumors avoid detection by the immune system. It is believed that by targeting PD-L1, MEDI4736 may block this ligand from sending out signals to T-cells to ‘ignore’ tumor cells, thereby countering cancer’s immune-evading tactics.
back to top 

GSK, formerly Glaxo SmithKline, and the Philadelphia Foundation have announced a $5 million grant package available to non-profits which will lead to healthier lives.
It’s called the GSK IMPACT Grant. IMPACT stands for Innovative, Measured, Partnered, Accountable, Community Centered and Transformative. Katie Loovis is GSK’s director of Community Relations.
back to top 

DATE: June 13, 2014, 8:30 a.m.-5 p.m.
LOCATION: Institute for Bioscience and Biotechnology Research, University of Maryland, in Rockville, MD
Emerging Strategies for the Production and Characterization of Biosimilars
The biomanufacturing industry faces an unprecedented challenge with the emergence of biosimilars. The pathway to approval for biosimilars is a fluid process and several key aspects are still not determined. The University of Maryland and the National Institute of Standards and Technology (NIST) will deliver a one-day symposium on the current trends of characterization and production of biosimilars. On Friday, June 13, 2014 at 8:30 am join the thought-leaders, policy-makers, and creators of biosimilars as we present current trends, ideas, and predictions.
back to top 
 June 11-12, 2014 June 11-12, 2014
Find out more about Strand Life Sciences here.
Learn about the current and future initiatives of the International Collaboration for Clinical Genomics (ICCG)! As part of our commitment to data sharing and collaboration to improve patient care, ICCG is creating a universal, clinical-grade database of genomic variation (including structural and sequence variants), available to the public through resources such as NCBI’s ClinVar database, and much more. We encourage the attendance of clinical laboratory personnel (from both cytogenetics and molecular communities), clinicians, genetic counselors, and others that are interested in setting the standards for clinical-grade databases of genomic variation.
back to top 

PRESENTER:
Karen Ambrose, Program Director, Business, Professional Licensure and Certification Programs
Montgomery College, Workforce Development and Continuing Education
ABSTRACT:
Montgomery College has joined with the world renowned Kauffman Foundation in support of current and future business owners before, during, and after the start-up process. Our plethora of courses is designed to meet the entrepreneurial needs of the aspiring business person and those who dream of business ownership.
Free monthly program offered through the Gateway to Innovation: Montgomery County Welcome Center for Federal and Academic Tech Transfer. For more information and additional calendar items, please visit TechTransferConnection.com.
Engage with others in the tech transfer field by joining the Gateway to Innovation LinkedIn Group.
back to top 

MdBio Foundation is looking for solutions to build a diverse and highly qualified STEM workforce. Read the latest from CEO Brian Gaines published in the Washington Business Journal.
Google turned heads last week when it disclosed that its workforce includes a troublingly low rate of women and minorities. We applaud Google for its leadership in sharing this data. The disclosure sparked national questions about the hiring practices at our most innovative companies.
back to top 

Johns Hopkins biomedical engineering students have designed a lightweight, easy-to-conceal shirtlike garment to deliver lifesaving shocks to patients experiencing serious heart problems. The students say their design improves upon a wearable defibrillator system that is already in use and should help persuade patients at risk for sudden cardiac arrest to wear the system around the clock.
“In two studies, up to 20 percent of patients who received the defibrillator garment that’s already available did not keep it on all the time because of comfort and appearance issues, problems sleeping in it, and frequent ‘maintenance alarms,’ which occur when the device does not get a good signal from sensors on the patient’s skin,” says Sandya Subramanian, who led the undergraduate team that built the new prototype. “We set out to address these issues and design a device that heart patients would be more likely to wear for longer periods of time—because their lives may depend on it.”
back to top 

Simeon Taylor, MD, PhD, a seasoned pharmaceutical executive who has served in senior positions with Eli Lilly and Bristol-Meyers Squibb, has joined the University of Maryland, Baltimore (UMB) as industry liaison with its Center for Clinical Trials and Corporate Contracts (CCT).
In his new role for CCT, he will assist faculty in developing research strategies that will maximize corporate partnering and funding opportunities, and the possible creation of valuable intellectual property. CCT is part of the University’s Office of Research and Development, and the part-time consultancy is funded in keeping with the University’s 2011-2016 Strategic Plan
back to top 

The U.S. National Institutes of Health has awarded $25 million to the J. Craig Venter Institute to back an initiative to study infectious diseases like malaria and influenza at the genetic level to help find better treatments and preventive measures.
The institute, with offices in Maryland and California, will use the 5-year grant from NIH to establish the Genome Center for Infectious Diseases to study the genetic secrets of a wide range of bacteria, viruses and parasites, officials said on Thursday.
back to top 

The University of Maryland, Baltimore County and the Chesapeake Innovation Center are teaming up to teach cyber entrepreneurs how to pitch to investors.
The seven-week program — Perfect Pitch — is scheduled to begin in September and conclude at the end of October. The program will end just before the start of the CyberMaryland Conference on Oct. 29.
back to top 

Early June is Health Datapalooza time, and this year’s event was again a whirlwind of energy, insights, and over 2,000 very motivated and passionate health data enthusiasts (health datapaloozers doesn’t sound right). The hallway conversations provided tons of of pragmatic, productive and engaging conversations, inspired by keynotes and presentations from Todd Park, Bryan Sivak, Jeremy Hunt, Atul Gawande, Steve Case, Jerry Levin, Vinod Khosla, Kathleen Seblius, Dwayne Spradlin, Francis Collins, Fred Trotter, and many more (if you haven’t heard of some of them, look them up, it’s worth your time).
back to top 

The University Economic Development Association (UEDA) is currently seeking nominations for its annual Awards of Excellence program, which recognizes cutting edge university-based economic development initiatives from across the country. The Awards of Excellence Program recognizes higher education institutions and their partners who are transforming their campuses into engines of economic prosperity through creative initiatives in five categories:
- Community Connected Campus: initiatives that promote the physical development of quality connected campuses and their surrounding communities;
- Research and Analysis: initiatives that enhance the capacity of colleges and universities to provide new forms of research and tools for community, economic and workforce development practitioners;
- Leadership and Collaboration: initiatives that support the development of collaborative economic development strategies and the leaders required to implement them;
- Innovation and Entrepreneurship: initiatives designed to support startups, high-growth companies and clusters within a region; and
- Talent Development: initiatives that promote the development of 21st-century skills.
back to top 

ERIC KING, 30, of Northern Liberties, is founder and CEO of Grand Round Table, a startup on 3rd Street near Market that develops software to help doctors make complicated diagnoses. GRT, which launched in 2012, is getting the correct diagnoses about 70 percent of the time. The company, which was part of the first DreamIt Health accelerator in 2013, has three full-time employees and has raised $110,000 from the Wharton Venture Initiation Program and Ben Franklin Technology Partners.
back to top 
PETER PRONOVOST: The biggest misperception is certainty. When you receive a diagnosis from a doctor, we don’t always know for 100% sure if it is correct. In reality, medicine is an inexact science. In 2012, Johns Hopkins researchers, including myself, found that in intensive-care units alone, diagnostic errors may account for as many deaths as breast cancer in the U.S.
This is part of a growing body of research highlighting the need to focus on diagnostic accuracy. We need to create health-care systems in which learning is incorporated into daily practice, so that physicians can receive feedback on the accuracy of their diagnoses. We can do this by standardizing care around best practices and standardizing data collection regarding clinicians’ diagnoses and the results they get: Health information technology makes this possible.
back to top 

In the translational medicine space — where medical research is “translated” into health tools and solutions for patients — the majority of engaged physicians are either biomedical researchers interested in advancing our understanding of the basic science, or are practicing doctors who want to improve clinical practice while focusing on patient care.
The number of physicians who receive training in understanding this translational research space is few, and even fewer are those who are able to take this skill set into the market to develop new technologies based off this understanding.
back to top 

At Medgadget we speak with quite a few physicians-turned-entrepreneurs, and one of the most enthusiastic and impressive we’ve known is Dr. Amy Baxter. We first met Dr. Baxter at the AARP conference in Atlanta last October where she was showing off a simple, yet effective tool she developed for pain relief called “Buzzy.” There’s been a lot of, well, buzz about the device ever since she pitched it on Shark Tank and turned down the investors. Informed by her experiences as a pediatric emergency physician, Dr. Baxter took time out of her schedule to answer a few questions we had about the device and why she thinks everyone who experiences pain should have one.
back to top 

A biotech entrepreneur wants to arm physicians with an electronic companion diagnostic tool to help hospitals reduce hospital-acquired infections.
Last year, antibiotic-resistant infections caused more than two million illnesses and 23,000 deaths in the U.S. alone, according to the Centers for Disease Control. About 70 percent of those antibiotic-resistant infections were caused by hospital-acquired infections.
back to top 

As the United States slowly emerges from the Great Recession, led by our cities and metropolitan areas, a remarkable shift is occurring in the spatial geography of innovation.
For the past 50 years, the landscape of innovation has been epitomized by regions like Silicon Valley — suburban corridors of spatially isolated corporate campuses, accessible only by car, with little emphasis on the quality of life or on integrating work, housing and recreation.
back to top 

Disruptive change is now a fact of life for many industries. Healthcare is no exception. Although healthcare has been changing for decades—think about the introduction of diagnosis-related groups (DRGs) or the initial push toward managed care in the 1980s—the Affordable Care Act (ACA) promises to accelerate both the rate of change and the level of uncertainty confronting the industry. Payors face navigating a difficult transition: from an industry in which the customer is often a corporation or small company and the business is paying claims to one in which consumers make healthcare purchasing decisions, the direct provision of care may be necessary for success, and consumer and retail capabilities really matter. Furthermore, payors must make this transition amid regulatory and consumer uncertainty and in a fairly short time frame. This industry and business-model shift is on a scale that few companies and few sectors in the economy have been through.
back to top 
Meet the humble “Magnetosperm,” a tiny, sperm-like robot with incredible potential in the medical world.
It’s approximately six times longer than a human sperm, and scientists at the American Institute of Physics say in a paper that Magnetosperm “technology could be used not only to help with fertilization, but also chemotherapy treatment.”
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 11-12
Bethesda North Marriott

June 11
Germantown Innovation Center

June 13
Institute for Bioscience & Biotechnology Research

June 15-19
Washington, DC

June 15-19
Washington, DC
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

GlaxoSmithKline ($GSK) has already made some moves to sell off some of its older meds. But now, it’s making more that could end up much, much bigger.
As Sky News reports, the pharma giant has solicited bids from a handful of private equity firms–Advent International, Blackstone and KKR included–for some or all of the 50 or so drugs in its established products portfolio, worth up to £7.5 billion ($12.6 billion). A spokesman for GSK told Sky the company is currently evaluating its options.
back to top 

Qiagen announced a collaboration with Eli Lilly and Co. to co-develop universal and modular assay panels for the simultaneous analysis of DNA and RNA biomarkers targeting multiple cellular pathways involved in common cancer types. The agreement includes the development of tests that will be based on Qiagen’s multi-modal, multi-analyte Modaplex analysis platform, which can process multiple sample types and biomarkers in a single test.
back to top 

Before coming into the program I would highly stress putting together a comprehensive product roadmap for the duration of the program as well as 2 months after. Start by splitting the upcoming 3 months into individual weeks & writing out goals both for the tech side (development, design, ux etc.) of the company as well as the business side (sales, fund raising, partnerships etc.). If possible have them written on a huge calendar for everyone in the company to see & be aware of.
Then go more granular and translate those weekly goals into individual tasks using any project management software, here I’d recommend Trello since its very intuitive, has great array of browser plugins to support different styles of task management & it’s totally free.
back to top 

June 10, 2014 08:00 AM – 10:00 AM
Bethesda North Marriott Hotel & Conference Center
Maryland is home to the most innovative and fast growing life science companies in the world. Two of these companies have recently made a huge splash – Amplimmune and GlycoMimetics. Hear from the CEOs of each company about why being homegrown and staying in Maryland is a key ingredient to success and what their achievements through IPO and acquisition can mean for your company and our life science community as a whole.
Discussion topics include:
- The challenges faced – the stories behind the stories
- Choosing your path and having the path choose you
- Planning for adaptation
- IPO vs. acquisition
back to top 

Epidarex Capital, a leading international venture capital firm, has raised over £47.5 million to lead investments in early-stage life science and health technology companies, including spin-outs from leading research universities. Global pharmaceutical company Eli Lilly and Company participated in the final closing with a significant capital commitment. King’s College London also invested in the final closing.
The fund’s early-stage focus is supported by a diverse range of investors, including four top research universities as well as the European Investment Fund, Scottish Enterprise and Strathclyde Pension Fund. Epidarex’s close working relationship with the Universities of Edinburgh, Glasgow and Aberdeen, three of Scotland’s top research universities, as well as with King’s College London, provides access to some of the most innovative healthcare start-ups, including those specialising in novel drug development.
back to top 

Imperial Innovations Group plc (AIM:IVO, ‘Innovations’), the leading technology commercialisation and investment company and S.R.One Ltd, GlaxoSmithKline’s venture captial fund, announce today that they have led, alongside co-investor UCL Business PLC (UCLB), an £850,000 seed financing for Puridify, a newly formed company providing purification solutions for biotherapeutic manufacturing.
Puridify’s technology was developed in the world-leading Advanced Centre for Biochemical Engineering at UCL (University College London). UCLB, the technology transfer office for UCL, licensed this technology to Puridify in 2014. It was the winner of the 2013 OneStart competition (founded by SR One and the Oxbridge Biotech Roundtable, http://www.oxbridgebiotech.com/onestart), which is designed to encourage young entrepreneurs. The company has received additional matched-funding awards totalling £780,000 from the UK’s innovation agency, the Technology Strategy Board, to support advancement of the technology.
back to top 

Immunotherapy, a new, promising therapeutic approach that leverages the power of the immune system to fight cancer, is the cornerstone of MedImmune’s oncology portfolio. As the biologics research and development arm of AstraZeneca, MedImmune is striving to push the boundaries of science to deliver life-changing medicines to patients.
By targeting the immune system, immunotherapy may lead to durable and prolonged response rates across a range of cancer types. Tumours are heterogeneous in that there many different cells that often mutate and grow in different ways. In lung cancer, for example, there may be more than 60 different mutations. Cancer often manages to continue replicating and becomes resistant and returns to growth, making it challenging to treat. This happens despite chemotherapy, surgery and other targeting therapies. Even worse, some of the existing therapies – especially chemotherapy – actually diminish the immune response, thus limiting the body’s own natural defence mechanisms to fight tumours.
back to top 
Bloomberg’s Drew Armstrong reports on the failed Pfizer and AstraZeneca merger on Bloomberg Television’s “Bloomberg Surveillance.”
back to top 

Register for the Spring National SBIR/STTR Conference and take your pick of 1-on-1 meetings with Agency principals on a first-come, first-serve basis. More than 300 meeting slots filled in a week at last year’s conference, so don’t delay. Once you register, you’ll receive access to descriptions of all 23 participating Agencies and Institutes to make your 1-on-1 meeting choices.
The conference, held at the Gaylord Convention Center in National Harbor, MD on June 16 – 18, will feature the SBA Administrator, representatives from all participating NIH institutes and key leaders from across the Federal government. The event is the nation’s largest annual gathering of SBIR/STTR Agencies, industry leaders, and awardees.
back to top 

The changing face of Frederick County’s economy is reflected in the rapidly growing field of bioscience and biotechnology companies locating or expanding here.
Agriculture and tourism may still be the leading economic factors in Frederick County, but the life sciences are becoming an integral part of the local economy. Additionally, many Frederick residents work for life science companies in Montgomery County and Washington. They may not be in the lab or doing administrative work for a bioscience firm in the county, but they benefit from the growth of local firms, as many of the companies collaborate on innovations and research.
back to top 

In this Hangout, we will discuss when a small business should contact the FDA, and what formal and informal mechanisms exist for device, drug, biologic, and diagnostic developers to obtain early advice from the FDA.
back to top 

Mobile devices are revolutionizing the health care system for patients and medical providers. Using this new technology wisely and securely is the goal of the health information technology experts who talked about trends in the field during a Health IT Forum at the Johns Hopkins University Montgomery County Campus.
The Health IT Forum series, held four times a year, is a community partnership co-sponsored by Montgomery County Department of Economic Development, the TechCouncil of Maryland and the Montgomery County Chamber of Commerce. The May forum, titled “mHealth: Bring Your Own Device,” attracted dozens of IT experts from the region.
back to top 

More than a decade has passed since scientists completed the Human Genome Project, a worldwide effort to decode our DNA. That DNA sequence contains instructions for making all of the proteins that our bodies need to function.
Now researchers have cataloged the vast majority of those proteins, creating a dynamic map of the human body called the proteome.
back to top 

The University of Maryland’s commitment to expanding science opportunities on campus could not be any more clear having now acquired a five-year, $1.2 million grant to further undergraduate science education from the Howard Hughes Medical Institute.
The College Park institution plans to use the grant to develop a new living-learning program for students enrolled in the biological and chemical sciences. It will include a residence hall with integrated support mentoring programs, community-building activities, co-enrollment in introductory science courses and advanced access to stellar hands-on research opportunities.
back to top 

Graduates and current tenants of Baltimore accelerator the Emerging Technology Centers contribute almost $174 million in direct economic impact in Baltimore, according to a new report commissioned by the city.
About 63 percent of the ETC’s 76 graduate companies have stayed in Baltimore and generate about $108.5 million in economic activity. Those 48 companies employ 311 workers.
back to top 

Can the nationally acclaimed Meyerhoff Scholars Program, which has an unparalleled record of advancing diversity in the sciences, be adapted successfully at more universities?
That question is at the heart of a new partnership between the Univ. of Maryland, Baltimore County (UMBC), the Pennsylvania State Univ., the Univ. of North Carolina at Chapel Hill (UNC), and the Howard Hughes Medical Institute (HHMI). The four institutions are launching the collaborative Meyerhoff Adaptation Project to learn whether elements of UMBC’s highly regarded Meyerhoff Scholars Program can be adapted at Penn State and UNC. The participating schools aim to learn what it takes to establish a successful program and to share what they learn so other institutions might follow.
back to top 

U.S. Sen. Barbara Mikulski says a new state roundtable on cyber security will focus on keeping the fast-growing industry’s jobs in Maryland, rather than going to Northern Virginia.
The Maryland Cybersecurity Roundtable will hold its first meeting on Thursday. The group includes Mikulski, Gov. Martin O’Malley, KEYW Corp. CEO Len Moodispaw and Jeffrey Wells, who oversees cyber for the Maryland Department of Business and Economic Development.
back to top 

A bipartisan initiative known as “21st Century Cures,” spearheaded by the House Energy and Commerce Committee, is aiming to keep the U.S. at the forefront of biomedical innovation.
The U.S. bio-phrama industry’s distinction as No. 1 hasn’t gone unnoticed in the rest of the world, says “A Path to 21st Century Cures,” a white paper that describes the initiatives main objectives. “[O]ther nations are now actively working to gain a competitive edge in various elements, whether through a focus on basic research or a streamlined approval process to bring new treatments to market more quickly,” according to the report.
back to top 

IAN READ, the chief executive of Pfizer, is not a man easily rebuffed. His attempt to woo AstraZeneca, a British drugs firm, included politesse—appearances before Parliament, web videos on Pfizer’s strategy—and, more importantly, a sweetened offer on May 18th worth around £70 billion ($120 billion). However, AstraZeneca’s board has rejected the bid and, as we went to press, it seemed dead in the water.
back to top 

After a lengthy and somewhat contentious markup, the House Science, Space and Technology Committee approved the Frontiers in Innovation, Research, Science, and Technology (FIRST) Act (H.R. 4186) by a vote of 20-16 on May 28th. As discussed in previous editions of Capitol Update, the bill reauthorizes and prioritizes federal investments at the National Science Foundation (NSF) and the National Institute of Standards and Technology (NIST) by funding research and development (R&D) to address national needs. The bill also sets priorities to drive the nation’s investments in science, technology, engineering and math (STEM) education programs.
The FIRST Act would require that the NSF publish a justification of each grant’s scientific merits and relevance to the broad national interest in order to meet minimum standards of public accountability and transparency in its grant funding decisions. The FIRST Act does not change NSF’s peer review process. Provisions of the FIRST Act also broadens the definition of STEM education to include computer science and supports student participation in nonprofit competitions, out-of-school activities and field experiences related to STEM.
back to top 

Once a year, all of the tech world stops to listen to what influential analyst Mary Meeker has to say. According to Meeker, current trends show a slight dip in internet user growth but she highlights that emerging markets are showing the fastest growth in terms of internet usage. This growth can be seen more rapidly in what she calls “difficult to monetise developing markets” like India, Indonesia and Nigeria.
Meeker, a partner at VC company Kleiner Perkins Caufield Byers (KPCB), unveiled her annual trends report at the Code Conference this week. The report highlights the disruption that is underway in areas like media consumption, education, healthcare, commerce, security, mobile and consumer apps. We have picked some key trends that we thought were worth highlighting.
back to top 

Microsoft Ventures, Microsoft’s startup investment arm, is to set up its first accelerator dedicated solely to companies in the cybersecurity area.
The four-month programme aims to provide startups with tools to succeed in business, from mentorship to help with development to marketing expertise. Companies that get through the program will have an opportunity to get $1m in funding, courtesy of Jerusalem Ventures Partners (JVP), which has partnered with Microsoft Ventures on the project.
back to top 

The Biotechnology Industry Organization today issued the following statement in response to Senator Patrick Leahy’s announcement that he will remove current patent legislation from the Senate Judiciary Committee’s agenda for now:
“BIO applauds Chairman Leahy’s decision to remove the controversial patent bill from the Senate Judiciary Committee’s agenda until greater stakeholder consensus can be achieved.
back to top 
All of us know that you have to be a little crazy to be an entrepreneur. Launching, let alone sustaining, a new enterprise can be challenging along almost every dimension − mentally, emotionally, and often financially. Historically, this reality has been even more sobering in the health care sector, where the typical hardships experienced by any start-up have been amplified by numerous industry-specific challenges: Extensive regulation, entrenched players with a strong grip on the status quo, confusing paths to entry, and an even more opaque path to payment have made health care a particularly treacherous territory for entrepreneurs.
back to top 

Numerous studies have shown that Americans of European origin have a more independent, and Asians have a more interdependent, social orientation, as measured by questionnaires asking about agreement with such statements as “I feel it is important for me to act as an independent person.” But this social-orientation difference is about 6 times greater among people from both backgrounds who are carriers of two particular alleles of a dopamine-receptor gene known as DRD4, according to a team led by Shinobu Kitayama of the University of Michigan. The alleles appear to predispose people to acquire behaviors that are considered socially desirable, the researchers say.
back to top 

Translating Ideas from Bench to Bedside
June 3rd, 2014, from 6 to 8:30 pm
Krieger School of Arts and Sciences Advanced Academic Programs
Johns Hopkins University Montgomery County Campus, Rockville, MD 9601 Medical Center Drive (A&R Building, Room 106-110)
Connect with physician entrepreneurs and other stakeholders in healthcare innovation as we explore the winning formula for successfully advancing new medical devices and technology to commercialization.
back to top 
 Join business leaders, government, elected officials and the media as we celebrate leadership that energizes our community and makes it possible to declare What’s Next is Here. Five prestigious awards will be presented including the Chairman’s Award. Witness the passing of the torch from the 2013-2014 MCCC Chair of the Board Christopher Carpenito, CPA, Executive Vice President & CFO, HESS Construction to the 2014-2015 MCCC Chair Lisa Cines, CPA, Office Managing Partner, Dixon Hughes Goodman LLP and welcome the 2014-2015 MCCC Board of Directors. Join business leaders, government, elected officials and the media as we celebrate leadership that energizes our community and makes it possible to declare What’s Next is Here. Five prestigious awards will be presented including the Chairman’s Award. Witness the passing of the torch from the 2013-2014 MCCC Chair of the Board Christopher Carpenito, CPA, Executive Vice President & CFO, HESS Construction to the 2014-2015 MCCC Chair Lisa Cines, CPA, Office Managing Partner, Dixon Hughes Goodman LLP and welcome the 2014-2015 MCCC Board of Directors.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

June 3
Johns Hopkins University Montgomery County Campus

June 5
Bethesda North Marriott Hotel & County Conference Center

June 11-12
Bethesda North Marriott

June 13
Institute for Bioscience & Biotechnology Research

June 15-19
Washington, DC
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Montgomery County, taking a page from the state’s playbook, has lured cybersecurity startup Mobile System 7 across state lines from McLean, Va. to Bethesda, Md. with $100,000 of investment and services according to The Washington Post. Expansion of the cybersecurity business sector has become a very high priority throughout Maryland and this is just the latest of a series of investments by the state and local governments to encourage successful cybersecurity companies to open or move businesses to Maryland.
The move is reminiscent of the way Luminal, a cybersecurity startup born in West Virginia, was enticed into moving to Frederick thanks to generous investment offers via the state government. That was state investment however, while the Mobile System 7 move comes after Montgomery County’s Department of Economic Development convinced the company that it would do better in Maryland than in Virginia.
back to top 

Supernus Pharmaceuticals, Inc. today announced that United Therapeutics Corporation UTHR +0.58% has paid Supernus a $2 million milestone payment under United Therapeutics’ license agreement with Supernus. This payment was due upon the launch of Orenitram™ (treprostinil) Extended-Release Tablets for the treatment of pulmonary arterial hypertension, in the United States. Orenitram™ utilizes a Supernus patented technology platform. In addition to the launch milestone, Supernus will receive royalties on net sales of Orenitram™, and may become entitled to additional milestone payments.
“We are pleased to have played a role in helping to bring Orenitram™ to patients and their physicians as an important new therapy option,” said Jack A. Khattar, President and CEO of Supernus. “Over the life of the product, we expect to receive significant recurring royalty revenue from United Therapeutics’ commercialization of Orenitram™.”
back to top 

Also this week in Pfizer-related Maryland biotech news: Gaithersburg-based GlycoMimetics Inc. collected a $15 million payment from the pharma giant stemming from its license agreement for the sickle cell anemia drug rivipansel.
GlycoMimetics in 2011 inked the Pfizer partnership, worth up to $340 million, to develop rivipansel (then just called GMI-1070) as a treatment for a complication of sickle cell disease called vaso-occlusive crisis.
back to top 

AstraZeneca on Monday rejected a “final” $119 billion buyout offer from Pfizer, possibly sinking a pharmaceutical mega-merger that could have jeopardized jobs at AZ’s Maryland subsidiary, MedImmune.
Pfizer, which would have created the world’s largest drug company through the deal, on Sunday evening increased its earlier $106 billion cash-and-stock bid. In a statement, AstraZeneca Chairman Leif Johansson called that new offer “inadequate” and said it would have “serious consequences for the company, our employees and the life-sciences sector in the U.K., Sweden and the U.S.”
back to top 

The second annual InvestMaryland Challenge, an early-stage business competition of the Maryland Department of Business & Economic Development, came to an exciting close Monday evening at the National Aquarium in Baltimore.
Maryland Governor Martin O’Malley, joined by DBED Secretary Dominick Murray, entrepreneurs and business leaders, applauded the winners of nearly $1 million in grants and prizes.
back to top 

A health IT startup launched last year by four recent Johns Hopkins graduates and one soon-to-be JHU graduate was one of four firms to win a $100,000 top prize Monday in the InvestMaryland Challenge, a state-run competition to help promising new companies in the life sciences and high tech industries.
The competition, run by the Maryland Department of Business and Economic Development, had 260 entries. Healthify took first place in the General Industry category, open to companies anywhere in the U.S. if they open a location in Maryland. Healthify, based in New York, beat out two other finalists—a wholesale food distribution website with a focus on local, sustainably-produced items; and an ad technology company.
back to top 

Engineering is a field with the power to transform lives across the globe, often in the most underserved regions. Nowhere is this maxim more apparent than at the Center for Bioengineering Innovation & Design (CBID), founded in 2007 at the Johns Hopkins University’s Department of Biomedical Engineering.
Each summer, teams of new engineering graduate students travel overseas to areas where poverty and poor healthcare dominate. They look for opportunities to put their skills to work solving difficult, and often deadly, health problems.
back to top 

QIAGEN N.V. (NASDAQ: QGEN; Frankfurt Prime Standard: QIA) today announced the expansion of its industry-leading portfolio of bioinformatics solutions with additional content from BIOBASE, a provider of expertly curated biological databases, software and services. With access to HGMD, an especially in clinical markets widely used biomedical data resource as well as to other unique content, QIAGEN expand its world’s most comprehensive, high-quality and up-to-date literature source for clinical research and diagnosis – further strengthening its market-leading position in the analysis and interpretation of sequencing data. QIAGEN’s growing bioinformatics and next-generation sequencing (NGS) franchises is positioned to benefit from the integration of BIOBASE, its assets and employees and will benefit the expansion of relationships with thousands of clinical labs and NGS users in life sciences.
“The ability of next-generation sequencing to rapidly deliver genomic insights is opening up new frontiers for clinical research and medicine, and QIAGEN is strategically addressing customers’ needs to interpret the massive amounts of data generated by NGS. With HGMD and other content from BIOBASE, a respected organization with a dedicated team and robust line of unique databases and software, QIAGEN is further extending its competitive advantage as the overall market leader for clinical interpretation of human sequencing data,” said Peer M. Schatz, Chief Executive Officer of QIAGEN. “Already today, more than 15,000 users worldwide rely on QIAGEN’s bioinformatics products for interpretation – and have processed over a quarter of a million genome sequences. HGMD is a unique fit with our offering and will integrate well. We expect to drive additional adoption of this leading literature-based knowledge base used by clinical reference labs for annotating hereditary variants, as well as BIOBASE’ other solutions by having integrated them into interpretation solutions shared with our Ingenuity Knowledge Base – adding value for QIAGEN and BIOBASE customers and accelerating our growth drivers in NGS and bioinformatics.”
back to top 

Biotech is certainly a growing industry in Greater Baltimore, and here’s more proof. These 10 companies all said they are growing their staff in the coming months, when we asked in our annual industry survey. Here are all the jobs they’re already looking to fill:
back to top 
 Tuesday, May 27, 2014, 08:30am – 02:00pm Tuesday, May 27, 2014, 08:30am – 02:00pm
SBIR/STTR programs promote small business innovation and profitability while simultaneously meeting the government’s research and development needs. Every year, small businesses receive millions of dollars in SBIR/STTR funds for research, development and commercialization purposes. This course will provide attendees with an overview of the SBIR/STTR programs; funding sources and eligibility requirements; best practices in SBIR/STTR proposals writing, involvement, and commercialization; and a discussion of how to protect your company’s legal interests in either program.
back to top 
 Join members from NHLBI’s SMARTT team for a free webinar to hear how three investigators utilized SMARTT services to advance the development of their lead candidates. Join members from NHLBI’s SMARTT team for a free webinar to hear how three investigators utilized SMARTT services to advance the development of their lead candidates.
Webinar agenda
- Overview of SMARTT
- Translational Investigator presentations:
- Steven Idell, MD, PhD, Vice President for Research at The University of Texas Health Science Center at Tyler
Development of fibrinolysin single chain urokinase plasminogen activator (scuPA) for pleural effusions
- Athan Kuliopulos, MD, PhD, Professor of Medicine at Tufts University and Tufts Medical Center
Development of PZ-128 for the prevention of arterial thrombosis in acute coronary syndrome and percutaneous coronary intervention
- Barry Coller, MD, Vice President for Medical Affairs and Physician-in-Chief, The Rockefeller University
Development of novel small molecule platelet inhibitors for pre-hospital treatment of myocardial infarction.
- Challenges in Early Translational Science: Industry Perspective
- Q&A with SMARTT
Representatives from NHLBI staff and from SMARTT’s regulatory, manufacturing, and pharmacology/toxicology facilities will be available to answer questions.
back to top 

Translating Ideas from Bench to Bedside
June 3rd, 2014, from 6 to 8:30 pm
Krieger School of Arts and Sciences Advanced Academic Programs
Johns Hopkins University Montgomery County Campus, Rockville, MD 9601 Medical Center Drive (A&R Building, Room 106-110)
Connect with physician entrepreneurs and other stakeholders in healthcare innovation as we explore the winning formula for successfully advancing new medical devices and technology to commercialization.
back to top 

City innovation economy classifications and rankings, 2014.
World’s largest city classification and global ranking with 445 benchmark cities classified, and all cities analyst ranked this year. Measuring each cities potential as an innovation economy at the current time, since 2007.
Based on 2thinknow analyst interpretation of 162 city indicators from 2thinknow City Benchmarking Data set.
back to top 

In almost every case, the scientist-entrepreneurs approaching LSN are falling victim to one or more fallacies that are propagated through the industry. LSN is in a dialogue with over 5,000 investors around the world, and the reality is that what many entrepreneurs believe to be sound business logic could be dooming their companies. This article compiles the top 10 fundraising misconceptions so that you can avoid these pitfalls.
back to top 

Providing shared space, services, equipment, and expertise to budget-conscious scientist-entrepreneurs who are looking to prove a hypothesis and launch a company is the mission of life science incubators. And they have helped many start-ups.
However, increasingly, scientist-entrepreneurs are disappointed with what they’re finding. LSN hears a lot of complaints because we are in dialogue with a lot of the firms that populate these incubators. They acknowledge that incubators are less expensive than going it alone. Still, they say the lab space is too expensive, the promise of seeding seasoned players who can augment the founding team often goes unfulfilled, and there’s little to no tactical fundraising support.
back to top 

American entrepreneurship is apparently on the decline, according to a recent article from FiveThirtyEight, but small business owners report consistently high levels of satisfaction with their choices despite the financial difficulties that they have faced.
“Americans started 27 percent fewer businesses in 2011 than they did five years earlier, according to data from the Census Bureau,” writes Ben Casselman on FiveThirtyEight. As a share of all companies, startups have been declining for more than 30 years.”
back to top 

Vinod Khosla, sassy VC and legendary co-founder of Sun Microsystems, has predicted the future of health.
In essence, he has said, our medical lives will become increasingly automated, with ultra-intelligent systems prescribing fine-grain recommendations to nurse us back to health. According to a newly released report, he predicts:
back to top 

The Angel Capital Association (ACA) today launched a campaign to protect startups from a devastating loss of angel capital if the Securities and Exchange Commission (SEC) increases the financial qualifications for accredited investors. The “Protect Angel Funding” initiative is a call to action to the startup ecosystem—from angel investors to economic development organizations and entrepreneurs – to urge regulators to preserve the definition of who qualifies as an accredited angel investor. Protecting this important investment class is critical to preserving the economic fuel it provides to startups and job creation. If changes proposed by “investor protection” organizations are enacted by the SEC, nearly 60 percent of angel investors would be eliminated.
The Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 requires the SEC to review the definition of an accredited investor in 2014 to determine whether it should be modified “for the protection of investors, in the public interest and in light of the economy.” Currently, an individual accredited investor is defined as someone with $1 million in net worth excluding the value of a primary residence, or annual income of $200,000. At issue is whether these financial thresholds should be arbitrarily raised based on inflation.
back to top 

In the US, many entrepreneurs see grants as “free money,” since they are not loans and don’t have to be repaid. A grant is not an equity investment, so the entrepreneur doesn’t have to give up a stake in the company either. Typically they can be used to fund product development and commercialization that would otherwise require outside investors.
A good place to start looking is the Small Business Innovation Research (SBIR) program, which is a lifeline for high-tech startups. A more general approach is to check out Grants.gov, which is a searchable directory of more than 1,000 federal grant programs. An advanced search tool is provided to search for a grant by eligibility, by issuing agency, or category.
back to top 

The DoD SBIR 2014.2 solicitation is now available for the submission of proposals.
Participating Department of Defense (DoD) agencies:
- Army
- Navy
- DARPA
- DLA
- DMEA
- Missile Defense Agency (MDA)
IMPORTANT DATES and DEADLINES
May 23, 2014
Solicitation opens and DoD begins accepting proposals
June 11, 2014
SITIS closes to new questions
June 25, 2014 6:00 am EST
Solicitation closes to receipt of proposals at 6:00 AM EST-plan ahead and submit early.
back to top 
Two year’s ago this month the Fourth Economy team completed an assignment for the Pennsylvania Life Science Leadership Advisory Council. At a news event in May 2012 we participated in the release of “Life Sciences Leadership for the Next Decade: Nurturing a Life Science Ecosystem for Job Creation and Economic Development in Pennsylvania”. This report highlighted five steps and related actions that the life science community could undertake to maintain the economic impact of the life science industry in the state.
Flash forward two years and we wanted to check in and see how the industry was performing in terms of employment and establishments. The following information summarizes our findings. The life science industry continues to be a strong overall sector for the state’s economy.
back to top 

Take a guess as to which industry suffered the most cybersecurity breaches in 2013.
According to one health IT startup, that’s healthcare. Protenus pointed that out at DreamIt Health Baltimore Demo Day in April.
As cofounder Robert Lord explained at Demo Day, electronic medical records, shockingly enough, “weren’t built with security in mind.”
back to top 

Its no secret: the University of Colorado receives hundreds of millions of dollars in federal funds that sponsor our research. My lab alone racks up expenditures of $800,000 a year to pay for post-doc and graduate student salary, tuition, materials and travel. Isn’t it preposterous to now ask the public for some more of their hard-earned cash as we, and many other universities now, do on CU Boulder’s new initiative?
It helps to first think about why the government actually does make these payments in the first place: to educate the next generation of engineers and scientists by working on research problems with important societal benefits. With this money becoming more competitive, the pool of ideas actually being supported becomes smaller with little to no feedback from the general public. Crowdfunding research allows the public to turn this around by enabling scientists to find support for projects currently not (or never) on the radar of conventional funding agencies, yet enjoying sufficient public interest. This category of research is probably the most obviously deserving of crowdfunding and has already enabled a host of documentaries, research excursions, and books, often by providing exclusively ideological or intellectual reward.
back to top 

QIAGEN N.V. (frankfurt prime standard:QIA) today announced that its therascreen® KRAS RGQ PCR Kit (therascreen KRAS test) has received U.S. Food and Drug Administration (FDA) approval to guide the treatment of metastatic colorectal cancer patients with Amgen’s Vectibix® (panitumumab). This marks the third FDA approval of a companion diagnostic from QIAGEN that has been paired with a novel medicine.
QIAGEN’s growing menu of clinically validated companion diagnostics is driving global dissemination of personalized healthcare, which uses genomic information to guide treatment decisions in individual patients.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

May 27
bwtech@UMBC Research and Technology Park

May 28
Online Webinar

June 3
Johns Hopkins University Montgomery County Campus

June 11-12
Bethesda North Marriott

June 13
Institute for Bioscience & Biotechnology Research
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 BioHealth Innovation, Inc. (BHI) announced this week its selection of George Jiang, M.D., M.B.A., PMP, as its new Chinese-American Entrepreneur-in-Residence (EIR). Dr. Jiang, a consultant with over 15 years of experience in life science research and development and in health industry business development, will lead in the scouting of technologies and partnership opportunities in life science hubs in the U.S. and China. BioHealth Innovation, Inc. (BHI) announced this week its selection of George Jiang, M.D., M.B.A., PMP, as its new Chinese-American Entrepreneur-in-Residence (EIR). Dr. Jiang, a consultant with over 15 years of experience in life science research and development and in health industry business development, will lead in the scouting of technologies and partnership opportunities in life science hubs in the U.S. and China.
“We welcome Dr. Jiang as our newest addition to the EIR program,” said Rich Bendis, President & CEO, BioHealth Innovation, Inc. “There is a vast opportunity to develop strategic partnerships with more Chinese corporations, and Dr. Jiang – as a Chinese American with experience as a consultant evaluating life science opportunities in China for a U.S. government agency – is in an ideal position to facilitate these collaborative efforts and the movement of new technologies along the commercialization path.”
Dr. Jiang will be based at BHI headquarters in Rockville, Maryland. BHI and China Fortune Land Development Co., Ltd. (CFLD) established this EIR position in February 2014. After the two organizations met during a four-city mission trip to China for leaders from Montgomery County and the DC metropolitan area, led by Ike Leggett, County Executive of Montgomery County, Maryland.
Dr. Jiang has facilitated biohealth product development across the academic, government, private and military sectors. As a senior consultant at Booz Allen Hamilton for eight years, Dr. Jiang has health industry experience in business development, subject matter expertise, stakeholder partnerships, and project/personnel management. Previously, Dr. Jiang served as the Director of Primate Immunology at the Naval Medical Research Center. Prior to that, he was a senior microbiologist at the University of Texas Health Science Center at San Antonio. He earned his M.B.A. from the Johns Hopkins University, PMP (project management professional certification) from the Project Management Institute, M.D. from Southern Medical University in Guangdong, China, and M.S. from the Beijing Institute of Basic Medical Sciences in China.
BHI and CFLD, a company specializing in investment and operation of industrial areas in China, including science parks, created the new Chinese-American Entrepreneur-In-Residence role to:
- Evaluate life science technologies in China and U.S. markets,
- Provide a strategic plan for start-ups,
- Advise on new ventures, and
- Lead commercial strategy for mature assets.
Dr. Jiang will be BHI’s 5th named EIR since the program began two years ago.
The organization is also actively recruiting for a China-USA EIR Program Intern to assist Dr. Jiang in his activities.
For questions, please contact Rich Bendis, President & CEO, 301-637-6439, rbendis@biohealthinnovation.org
back to top 

Guest Speaker: Rich Bendis, Founder, President & CEO, BioHealth Innovation, Inc.
The chair-elect of the Economic Development Committee, Leslie Weber, welcomed MCCC members and introduced the guest speaker, Rich Bendis, Founder, President & CEO, BioHealth Innovation, Inc. (BHI). BHI is an innovation intermediary that connects market relevant research assets to appropriate funding, management, and global markets to facilitate the development of commercially viable biohealth products and companies.
The organization co-founds early stage biohealth companies and works directly with existing regional commercially relevant biohealth firms on their early business development milestones to accelerate commercialization. Ninety percent of the companies BHI is working with are in Montgomery County Maryland.
back to top 
 BioHealth Innovation, Inc. (BHI) is seeking a highly talented professional to serve as its China-USA EIR Program Intern. The China-USA EIR Program Intern will support the Chinese-American Entrepreneur In Residence (EIR) position, which manages the relationship between BHI and its partner, China Fortune Land Development Co., Ltd. The China-USA EIR Program Intern will primarily support the EIR through research and translation, but will also perform administrative tasks. BioHealth Innovation, Inc. (BHI) is seeking a highly talented professional to serve as its China-USA EIR Program Intern. The China-USA EIR Program Intern will support the Chinese-American Entrepreneur In Residence (EIR) position, which manages the relationship between BHI and its partner, China Fortune Land Development Co., Ltd. The China-USA EIR Program Intern will primarily support the EIR through research and translation, but will also perform administrative tasks.
More Information
back to top 

William E. (Brit) Kirwan, chancellor of the University System of Maryland and a longtime national figure in public higher education, will resign from his leadership post when a successor is named, Mr. Kirwan announced on Tuesday.
Mr. Kirwan, who is 76, has led the Maryland system for 12 years and served as president of Ohio State University from 1998 to 2002. He has been at the forefront of national conversations about reducing college costs, including athletics spending.
back to top 
Mike Baader, the head of Venable’s Baltimore office, will be joining the law firm’s longtime client, Greenspring Associates, as a partner and general counsel, sources said.
The sources said Baader will stay on with the firm for several months to assist in the transition. Courtney Capute has been named to succeed Baader as head of the law firm’s Baltimore practice.
back to top 

Pharmaceutical behemoths New York-based Pfizer and London-based AstraZeneca are waging a trans-Atlantic battle over a potential multibillion-dollar buyout that has put both companies and their respective governments on the defensive.
The kerfuffle raises questions about the future of 3,100 scientists and manufacturers employed in the Maryland offices of MedImmune, a biotechnology company that local officials worry could be gutted if its corporate parent, AstraZeneca, gets bought.
back to top 

Pfizer has again raised its offer for AstraZeneca, making what it said was a final effort to bring the giant British drug maker to the negotiating table.
The latest offer, made Sunday evening, is worth about $119 billion. It comes after AstraZeneca’s rejection of several private and public offers from Pfizer.
back to top 

Join us at another BioBuzz event with Sponsor The UMD Biotechnology Research and Education Program (BREP) on May 21st from 5:00 – 7:30 p.m. at American Tap Room in Rockville, MD. .This location is a short walk from the Metro in the Rockville Town Center. We’re capping the registration at 120 people, so make sure you sign up today!
back to top 

Cybersecurity is a major growth engine in the region. Get on board by joining over 200 cyber leaders from the private, government and academic sectors at the 2014 CyberMontgomery Forum. Plus, hear a keynote address by The Honorable Thomas J. Ridge, the first Secretary of the U.S. Department of Homeland Security and former two-term Governor of Pennsylvania.
Attendees will also get an update on the National Cybersecurity Center of Excellence and hear from other industry experts and guest speakers including Congressmen Ben Cardin and Chris Van Hollen, Montgomery County Executive Isiah Leggett and representatives from Lockheed Martin, Discovery Communications, DMI, Triumfant, Koolspan, Inc. and many more.
back to top 
 Wednesday, May 14, 2014, 06:00pm – 08:30pm Wednesday, May 14, 2014, 06:00pm – 08:30pm
Health Care Providers: Learn About the First Rx-only, FDA-Cleared, Mobile Therapy for Type 2 Diabetes
Smartphones and tablet computers are a new way to deliver diabetes therapy. The Food and Drug Administration (FDA) calls this new type of therapy MPT: “mobile prescription therapy.” MPT products tell you what to do to take care of your disease. The advice shows up on your smartphone or other device.
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association for life science and technology, today announced the winners of its 26th Annual Industry Awards. More than 700 technology and business leaders from around the state attended the celebration last night, which took place at the Bethesda North Marriott Hotel & Conference Center.
“The individuals and companies we honored at last night’s industry awards celebration represent the tremendous talent, innovation and dedication we have in Maryland’s thriving technology community,” said Phil Schiff, TCM’s CEO. “The nominees for this year’s awards were truly outstanding, and exemplify how Maryland’s technology community is playing a vital role in ensuring our state’s long-term prosperity.”
back to top 

Cybersecurity will be a major growth engine in the region for many years to come. The 2014 CyberMontgomery Forum is YOUR chance to join with cyber leaders from the private sector, government and academia. Hear from The Honorable Thomas J. Ridge, the first Secretary of the U.S. Department of Homeland Security and former Governor of Pennsylvania.
Plus, get an update on the National Cybersecurity Center of Excellence and hear from industry experts and guest speakers including Congressmen Ben Cardin and Chris Van Hollen, Montgomery County Executive Isiah Leggett and representatives from Lockheed Martin, Discovery Communications, DMI, Triumfant, Koolspan, Inc. and…
back to top 

GlycoMimetics, Inc. announced today that data from a Phase 2 clinical trial of its lead drug candidate rivipansel ( GMI-1070 ) in pediatric patients has been presented at the American Society of Pediatric Hematology Oncology (ASPHO) 27th Annual Meeting . As part of the symposium entitled, “Sickle Cell Disease: Saving the Brain and Treating the Pain,” Timothy McCavit, M.D., M.S., Assistant Professor of Pediatric Hematology-Oncology at UT Southwestern Medical Center, highlighted the results observed in a randomized, multi-center, double-blind, adaptive Phase 2 study of rivipansel in pediatric sickle cell disease patients with vaso-occlusive crisis (VOC). The ASPHO Meeting is taking place May 14 through May 17 at the Palmer House Hilton in Chicago.
“Because sickle cell disease is genetic with symptoms presenting in patients at very young ages, there is a huge unmet need for managing its symptoms in pediatric populations. Dr. McCavit’s presentation at the ASPHO meeting underscores the potential role of rivipansel in addressing VOC in pediatric patients,” said Helen Thackray, M.D ., Vice President of Clinical Development and Chief Medical Officer at GlycoMimetics. “We remain committed to supporting the advancing study of this potential therapy in both children and adults and look forward to continued research through the Phase 3 study, which will be led by our collaborator Pfizer, Inc.”
back to top 

OpGen, Inc., a Gaithersburg based molecular diagnostics company, announced today that it has launched a new molecular-based test that can quickly and reliably identify patients at risk for harboring serious disease-causing microbes that can resist even the strongest antibiotics. The Acuitas™ MDRO Gene Test is a comprehensive molecular screening tool that can directly detect as many as seven genes from one patient sample, and will help hospitals and public health officials combat some of the most critical multi-drug resistant organisms (MDROs) threatening patients in healthcare facilities.
“Drug-resistant ‘superbugs’ pose a serious and immediate threat to the world’s health and safety increasing the likelihood of prolonged illnesses, higher costs – even death,” said Evan Jones, Chairman and CEO, OpGen, Inc. “The new Acuitas MDRO Gene Test makes MDRO screening more efficient and cost-effective by delivering comprehensive, precise and actionable information to healthcare practitioners within 24 hours, assisting them in their efforts to combat and prevent the spread of these complex and potentially life-threatening infections.”
back to top 

Richard Griffin, Director of Economic Development for the City of Frederick, and Julie Garner, Director of External Affairs, Maryland for AstraZeneca, accepted the award at the 2014 MEDA Awards Ceremony. The ceremony annually honors “standouts” in the field of economic development and the professionals who foster MEDA’s success today.
The award-winning plant, located next to the original 163,802 square foot biomanufacturing facility, provides increased capacity and has enabled AstraZeneca and MedImmune to meet the demand for new products. The company boasts world-class protein engineering and process development capabilities.
back to top 

“Hi, my name is Krzysztof Sitko, cofounder of Aegle, and I’m going to tell you today about how we failed throughout all of DreamIt Health, and why you might consider investing in us anyhow.”
A month ago, that wasn’t how I pictured my presentation at our startup accelerator’s Demo Day. I was working tirelessly alongside my cofounders to put together a business. Yet there I was in front of a room of 200 to announce that we didn’t have a market for the product we had been working on.
back to top 

THIS MOBILE HEALTH TOOL HELPS PEOPLE TO BE ABLE TO CONFRONT THEIR ANXIETIES AND THEIR FEARS.
A new self-help tool has now been developed by a team of psychologists, physicists, and developers, which uses augmented reality over a mobile health platform, with the goal of helping users to be able to treat their phobias and overcome their anxieties.
back to top 

Maryland lawmakers have penned a letter to Pfizer asking the New York-based pharmaceutical giant to ensure that it will not shed jobs at Gaithersburg-based MedImmune should it succeed in acquiring parent company AstraZeneca.
Pfizer has offered as much as $106 billion to acquire United Kingdom-based AstraZeneca in a deal that could rank among the largest ever for the pharmaceutical industry. So far, the companies have not agreed on a purchase price.
back to top 

San Diego-based biopharmaceuticals developer Lumena Pharmaceuticals has been acquired by UK-based Shire plc, in a deal worth $260M plus earn out, the companies said this morning. Shire said the buy would help add to its rare diseases portfolio. Lumena Pharmaceuticals had filed for an IPO in April; the company was venture backed by Alta Partners, New Enterprise Associates, RiverVest, A.M. Pappas Life Sciences Ventures, and Adage Capital Partners.
back to top 

The recently published “Johns Hopkins Guide to Digital Media”, weighing in at over five hundred pages, claims to be the first “systematic and comprehensive” reference work on digital media. However, the reader need only scan the table of contents with entries ranging from the expected, such as “Blogs,” to the obscure such as “Searle’s Chinese Rooms,” to appreciate that it is neither systematic nor comprehensive. And, this is not necessarily a bad thing. For, The Guide does indeed accomplish what it sets out to do: provide readers with ” a GPS and a map of the territory of digital media, so that they will be able to design their own journey through this vast field of discovery.”
back to top 

University of Maryland is well-known across the nation and internationally as a forward-thinking research institution, which is why it wasn’t too surprising to hear that UMD is now home to one of the nation’s fastest university-owned supercomputers.
Put simply, the supercomputer going by the name of Deepthought2 (appropriate, eh?) will help to support advanced, high-performance computing and research with the ability to do things like simulate fire and combustion. The contraption has a processing speed of about 300 teraflops, which is crazy fast. How fast? Absurdly speedy.
back to top 

Johns Hopkins announced on May 6 its plans to build a new cancer treatment building in Baltimore. The facility will be constructed with the help of a $65 million gift. It will be named after the late Albert P. “Skip” Viragh, Jr., a philanthropist and a former cancer patient treated at Johns Hopkins, who died in 2003, at the age of 62.
The Skip Viragh Outpatient Cancer Building is scheduled for completion in 2017. It will be constructed on the southeast corner of Fayette Street and North Broadway, in East Baltimore, and will feature about 50 exam rooms, advanced cancer imaging, a specially designed cancer diagnostic and treatment planning center, and many other facilities and services.
back to top 

A 300-teraflop supercomputer named Deepthought2 will now power research at the University of Maryland. Housed in the institution’s new 9,000-square-foot Cyberinfrastructure Center, Deepthought2 was developed with high-performance computing solutions from Dell and will support advanced research activities ranging from studying the formation of the first galaxies to simulating fire and combustion for fire protection advancements.
Deepthought2 replaces its namesake Deepthought, installed in 2006. According to a university statement, “the new supercomputer is 10 times faster than its predecessor, able to complete between 250 trillion and 300 trillion operations per second. It has a petabyte (1 million gigabytes) of storage and is connected by an InfiniBand network, a very high-speed internal network. Put another way, Deepthought2 is the equivalent of 10,000 laptops working together, it has 2,000 times the storage of an average laptop, and its internal network is 50 times faster than broadband.”
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Join members from NHLBI’s SMARTT team on May 28, 2014 (1:00-2:30 pm EST) for a free webinar to hear how three investigators utilized SMARTT services to advance the development of their lead candidates.
Webinar agenda
- Overview of SMARTT
- Translational Investigator presentations:
- Steven Idell, MD, PhD, Vice President for Research at The University of Texas Health Science Center at Tyler
Development of fibrinolysin single chain urokinase plasminogen activator (scuPA) for pleural effusions
- Athan Kuliopulos, MD, PhD, Professor of Medicine at Tufts University and Tufts Medical Center
Development of PZ-128 for the prevention of arterial thrombosis in acute coronary syndrome and percutaneous coronary intervention
- Barry Coller, MD, Vice President for Medical Affairs and Physician-in-Chief, The Rockefeller University
Development of novel small molecule platelet inhibitors for pre-hospital treatment of myocardial infarction.
- Challenges in Early Translational Science: Industry Perspective
- Q&A with SMARTT
Representatives from NHLBI staff and from SMARTT’s regulatory, manufacturing, and pharmacology/toxicology facilities will be available to answer questions.
back to top 

David Mott will speak at the BioTrinity 2014 Conference, the European Bipartnering and Investment Conference on May 12-14, 2014.
BioTrinity is the premier European Biopartnering and Investment Conference, partnering R&D companies with big pharma and more biomedical investors than any other key European partnering conference.
back to top 

The National Venture Capital Association on Wednesday named Scott Sandell, a Silicon Valley-based general partner with New Enterprise Associates, its next chairman.
He takes over as chair of the Arlington-based VC trade group at an optimistic time, at least compared with the years following the recession. By the NVCA’s own numbers: Venture firms, nationally, are seeing increasing success luring back limited partners, while a greater number of venture-backed companies are going public. Total VC dollars invested hit a more than dozen-year high last quarter.
back to top 

The National Institutes of Health announced on Wednesday a new policy requiring that both sexes be represented among the subjects of preclinical biomedical research it finances involving animal and cell models.
More than two decades after requiring gender balance among human beings in the trials themselves, NIH leaders said they now realize that the same step should be applied to the laboratory experiments that inform those trials.
back to top 

Every summer, the National Security Agency offers about 250 internships to undergraduate and graduate students interested in pursuing careers with the federal agency. If you can snag one, your career will be off to a great start.
But getting one is the hard part. There are more than 10,000 applicants every year for the internships, which span the science, technology, engineering and math fields. Richard Ledgett, deputy director of the NSA, on Wednesday told members of the BWI Business Partnership the selection process is grueling because of the high standards and required security clearances.
back to top 

Johnson & Johnson’s ($JNJ) Janssen arm is planning to open another biotech incubator, this time setting its sights on South San Francisco in hopes of finding a few promising drug developers.
The new operation, dubbed Janssen Labs @South San Francisco, will be a 30,000-square-foot mix of lab and office space with room for up to 50 startups, J&J said. Mirroring Janssen’s flagship San Diego incubator, the new facility will be staffed by some of J&J’s biotech brains and provide operational support, education and business services to its guest companies.
back to top 

The National Institutes of Health contracting arm has launched a $20 billion governmentwide acquisition program, Nextgov reports based on solicitation documents.
back to topa> 

RCT Ventures has just announced a new opportunity for early-stage medical device companies: the 2014 MedTech Innovator competition. Companies and university-stage teams are encouraged to apply. Ten semi-finalists will be selected by a panel of medical device venture capitalists that will receive free registration to the Wilson Sonsini Medical Device conference. There, a live audience will select the winner, who will receive the $100k grand prize.
RCT Ventures’ last contest, Medtech Idol, had a fascinating array of gadgety competition with Aventamed‘s impressive ear-tube placement device coming out on top. When asked about the competition, Paul Grand, Managing Director for RCT Ventures said, “In today’s lean start-up model, the $100k prize should be enough to allow the winner to bridge the funding gap and achieve a milestone that can help them secure a larger funding round or critical industry partnership.”
back to top 

D.C. startup incubator and co-working campus 1776 filed paperwork with the SEC to create a $25 million seed investment fund according to a report in the Washington Business Journal. The filing, issued in the name of “1776 Seed Investors, LP,” would be part of a longer term plan by 1776 to start funding startups while it continues to incubate them at its D.C. headquarters.
back to top 

Electronics giant Samsung recently announced a foray into big pharma. The South Korean company is set to invest over $2 billion into biopharmaceuticals—drugs developed from biological sources (e.g. vaccines or gene therapies) as opposed to traditional chemical cocktails—with a focus on creating cheaper versions of existing therapies.
But cheapness won’t be Samsung’s only advantage. The company better known for its smartphones could also take advantage of the fact that the pharma industry has been slow to explore mobile health technology.
back to top 

Researchers at the Mayo Clinic have completed a groundbreaking new test: They knocked widespread blood cancer into remission with a single massive blast of measles vaccine.
Stacy Erholtz, who was suffering from an advanced stage of blood cancer, recovered thanks to an intravenous injection of the measles virus, which was sufficient to overwhelm the cancer’s natural defenses, the StarTribune reports.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

May 20
Germantown Innovation Center

May 21-24
The Marriott Wardman Park DC

May 21
American Tap Room

May 27
bwtech@UMBC Research and Technology Park

May 28
Online Webinar
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
| | | | | | | | | |