|
|
|
 ROCKVILLE AND BALTIMORE, MARYLAND, September 30, 2014 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today that venture capitalist, Tania Fernandez, Ph.D., has joined the BHI team as a strategic advisor. Dr. Fernandez will be a member of the management team for a new BioHealth Gap Fund, which will provide up to $50 million in seed and early-stage equity investments to therapeutics, medical device, diagnostics, and health IT companies in Maryland. Additional BioHealth Gap Fund management team members include Richard Bendis, Ram Aiyar, Todd Chappell, and Ken Malone, who each bring domain knowledge and industry access to the fund. ROCKVILLE AND BALTIMORE, MARYLAND, September 30, 2014 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today that venture capitalist, Tania Fernandez, Ph.D., has joined the BHI team as a strategic advisor. Dr. Fernandez will be a member of the management team for a new BioHealth Gap Fund, which will provide up to $50 million in seed and early-stage equity investments to therapeutics, medical device, diagnostics, and health IT companies in Maryland. Additional BioHealth Gap Fund management team members include Richard Bendis, Ram Aiyar, Todd Chappell, and Ken Malone, who each bring domain knowledge and industry access to the fund.
“Dr. Fernandez has ten years of experience as a venture capitalist in the life sciences/biotechnology industry. Her work in Silicon Valley, along with her research experience at the National Cancer Institute, makes her a tremendous asset to the BHI team,” said Richard Bendis, BHI President & CEO. “Dr. Fernandez brings a West Coast investment perspective, and she will have an active role in helping to manage the BioHealth Gap Fund. She will also support our BHI Entrepreneurs-in-Residence and clients: helping our startups to grow and raise strategic funding.”
Dr. Fernandez previously was a venture capitalist with Burrill & Company, a $1.5 billion fund with a portfolio of 103 companies in the biotechnology/life science industry. Dr. Fernandez has over sixteen years of industry experience with the ability to successfully identify and assess promising scientific technologies/products and business models for venture investments across the sectors of therapeutics, diagnostics, next-generation sequencing and healthcare delivery. She has led and managed investments through both operational and Board positions. Roche acquired her lead investment, Bioimagene, for $100 million within two years of investment. Dr. Fernandez plays an active role in training and mentoring entrepreneurs in Silicon Valley and provides strategic advisory services to companies that range from startups to revenue-driven companies.
BHI’s BioHealth Gap Fund is anticipated to be a $50 million fund that will invest in disruptive companies throughout Central Maryland looking to close the gap between seed and early-stage financing. The fund will focus on therapeutics, medical devices, diagnostics, and health IT companies, and provide seed and early-stage equity investments along with follow-on capital for growth to help companies exit successfully.
“It is an honor to have the opportunity to work with BHI and to help manage the new BioHealth Gap Fund,” said Dr. Fernandez. “The potential to serve a pressing need in the current life sciences funding landscape in Maryland is tremendous, and I am delighted to be a part of it.”
About BioHealth Innovation, Inc.
BioHealth Innovation, Inc., is a regional innovation intermediary focused on commercializing market-relevant bio-health innovations and increasing access to early-stage funding in Maryland.
back to top 
 On September 10, 2014, Tasly Pharmaceuticals, Inc. (Tasly) and BioHealth Innovation, Inc. (BHI) signed a Memorandum of Agreement (MOA) which aims to advance their business partnership. The MOA also lists specific near- and long-term collaboration activities between the two parties. On September 10, 2014, Tasly Pharmaceuticals, Inc. (Tasly) and BioHealth Innovation, Inc. (BHI) signed a Memorandum of Agreement (MOA) which aims to advance their business partnership. The MOA also lists specific near- and long-term collaboration activities between the two parties.
back to top 

U.S. Senators Ben Cardin and Barbara A. Mikulski (both D-Md.) today announced that the Department of Labor (DOL) has awarded $14,957,899 in federal funding to fourteen Maryland community colleges as part of the Trade Adjustment Assistance Community College and Career Training (TAACCCT) initiative. The TAACCCT program allows community colleges and other institutions to expand their ability to provide quality education and job training programs in two years or less.
Of the nearly $15 million, Montgomery College received $5,371,743 to lead and fund the Cyber- Technology Pathways Across Maryland (CPAM) Consortium. CPAM is comprised of fourteen Maryland community colleges. It seeks to train and educate Trade Adjustment Assistance workers, veterans, the un- and –under employed and low skilled adults. The Consortium will work to connect participants with employers looking to fill thousands of unfilled job openings. CPAM focuses on bringing women and other underrepresented populations into the growing fields of cyber technology and cyber security.
back to top 
 Rich Bendis was interviewed on 99.1 WNEW Newstime about partnering with Roche and describing what it is that BioHealth Innovation does for Maryland. Rich Bendis was interviewed on 99.1 WNEW Newstime about partnering with Roche and describing what it is that BioHealth Innovation does for Maryland.
back to top 
 This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation. This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.
back to top 
 Tuesday, October 07, 2014, 08:00am – 10:00am Tuesday, October 07, 2014, 08:00am – 10:00am
At the forefront of innovation, the Consumer Electronics Association (CEA) unites 2,000 companies in the consumer technology industry and owns and produces the world’s largest annual innovation tradeshow, the International CES®. Gary Shapiro, the president and CEO of CEA, knows innovation and is the go-to source to tell you what’s cool in consumer electronics. Ask him what’s in this year and he’ll talk about Ultra HD 4K TV, 3-D robotics, and wireless health. He’ll also mention that the biggest drivers of consumer electronics are products that didn’t even exist a few years ago. Join Gary as he takes you on a spirited discussion about the importance of innovation in the U.S. economy, green technology, and keeping the American dream alive.
Get a look inside Shapiro’s passion for innovation and see what it’s like to be at the forefront of a multi-billion dollar industry.
back to top 

Tuesday, October 7, 2014 from 6:00 PM to 8:00 PM (EDT)
Rockville, Maryland
In February 2013, The Sunshine Act was included as the Transparency Reports and Reporting of Physician Ownership or Investment Interests section of the Patient Protection and Affordable Care Act (ACA). The Sunshine Act requires manufacturers of drugs, medical devices, and biologicals that participate in U.S. federal healthcare programs to report certain payments and items of value (typically $10 or more and totaling $100 annually or greater) given to physicians and teaching hospitals. Failure to stay in compliance may result in fines ranging from $10,000 to $1,000,000 annually. Whether you are a practicing physician or your startup has a medical device, drug, or related product, you are impacted by the ACA’s Physician Payments Sunshine Act. In July, CMS proposed removing the reporting exemption for any payments or transfers of value made to physicians who participate in accredited CME programs. We’ll talk with experts in compliance and policy who will share their experiences in implementing these new policies and how you can understand the implications of the law, dispute inaccuracies, and stay in compliance!
back to top 

Thursday October 9, 2014 9:00 AM – 3:15 PM
The DC I-Corps Fall 2014 Regional Cohort officially kicks off on October 9th at the Microsoft building in Chevy Chase, MD. Please register to join us for our Showcase lunch, in which successful teams from previous cohorts will present their businesses and discuss lessons learned from the I-Corps program.
Showcase agenda:
- Welcome and lunch
- Introduction of incoming Fall cohort teams
- Presentations by Accelerator teams
- Q&A
About DC I-Corps: DC I-Corps is a regional program designed to foster, grow and nurture an innovation ecosystem in the nation’s capital, the nearby states of Maryland and Virginia, and the mid-Atlantic region. The program is sponsored by the National Science Foundation (NSF) and jointly run by the University of Maryland College Park, George Washington University, Virginia Tech and Johns Hopkins University. The program provides real world, hands-on training on how to successfully incorporate innovations into successful products. The ultimate goal is to create a new venture or licensing opportunity for program participants.
back to top 

The National Institutes of Health and the U.S. Food and Drug Administration will receive a top national award for the year’s most outstanding intellectual property licensing deal, for technology transfer of a pioneering, low-cost meningitis vaccine launched in sub-Saharan Africa. The 2014 Deals of Distinction Award will be presented to the two federal agencies and their collaborators by the Licensing Executives Society at the society’s 50th annual meeting, Oct. 5-8 in San Francisco.
back to top 
Johns Hopkins University named Leslie Ford Weber director of campus, government and community affairs for Montgomery County in Rockville.
She succeeds Elaine Amir, who retired in September 2013.
Weber had been interim executive director of the campus since October and director of government and community affairs for Suburban Hospital in Bethesda since July 2011.
back to top 

Wednesday, October 1st, is the deadline to apply for the next round of BioMaryland Center biotechnology development awards. The BioMaryland Center is partnering this year with Maryland’s Dept. of Health and Mental Hygiene (DHMH) and the Center for Medical Technology Policy (CMTP) to incorporate improved health care quality and cost reduction criteria in the selection process for the Center’s annual Awards program.
A total of $1M will be awarded on a competitive basis to projects, $50,000-200,000 each, advancing technologies toward commercialization–with preference given to projects which improve patient outcomes and reduce costs.
BioMaryland, DHMH and CMTP will provide ongoing advice and support to the teams whose projects are selected for funding to address technical, scientific, regulatory and reimbursement issues that may be encountered during the development process.
back to top 

Nationally, young firms play a central role in the creation of new employment opportunities. High-tech companies are particularly important to job creation: over 9% of average annual net job creation from 1990-2011 is due to high-tech firms younger than 5 years old. All private firms younger than 5 years old created less than 6% of average annual net job creation.
Young, innovative companies have generated the majority of new jobs in Greater Baltimore over the past 5 years. This trend is consistent with similar metropolitan regions and the coun- try as a whole. New technologies, research, and ideas spawn new teams, divisions, and entire companies. Continuing to support and encour- age innovation is imperative to sustaining growth in the Greater Baltimore economy.
back to top 

Circulomics Inc has been awarded a Phase I Small Business Innovative Research (SBIR) grant by the National Institutes of Health (NIH) to develop its Nanobind DNA and RNA isolation technology for formalin-fixed, paraffin-embedded (FFPE) samples. This grant was made by the National Institute of Environmental Health Sciences to create a novel method for extracting molecular information from archived tissues. FFPE sample archives contain a wealth of molecular biomarker information that can be compared to standard histological analysis and correlated to clinical outcomes. However, the DNA and RNA isolated from FFPE samples are often degraded due to damage from the FFPE preservation process as well as contaminated by residual formalin and paraffin wax.
back to top 

Emergent BioSolutions Inc. (NYSE:EBS) today announced the initiation of the pivotal non-clinical efficacy study to demonstrate that BioThrax® (Anthrax Vaccine Adsorbed) manufactured at large scale in the company’s new modern facility, Building 55, is comparable to the BioThrax currently manufactured in its approved facility, Building 12. Data from this study will be used to support licensure of Building 55. BioThrax is the only vaccine licensed by the U.S. Food and Drug Administration (FDA) for the prevention of anthrax disease.
back to top 

ATCC increases focus on scientific reproducibility and leverages technological advances with new senior executive appointments
ATCC, the premier global biological materials resource and standards organization, is pleased to announce two important appointments to the senior leadership team. Barbie Bigelow has joined ATCC as Executive Vice President of Strategy and Technology and Dr. Maryellen de Mars also joins as the Senior Director for the Standards Resource Center. Both roles are new positions at ATCC.
back to top 

AstraZeneca announced today that its global biologics research and development arm, MedImmune, has received fast track designation from the US Food and Drug Administration (FDA) for its investigational monoclonal antibody (mAb) MEDI3902 for the prevention of nosocomial pneumonia caused by Pseudomonas aeruginosa (P. aeruginosa).
Pseudomonas aeruginosa (P. aeruginosa) causes serious disease in hospitalised patients. The FDA’s Fast Track programme is a process designed to expedite the development and review of drugs to treat serious conditions and fill an unmet medical need.
back to top 

There are two prevailing perceptions about innovation and start-ups: first, they are all tech driven, and second, they originate from just a few regions — chief among them, Silicon Valley. I’ve seen firsthand that innovation can happen anywhere, and that it is accelerating in places that typically don’t grab headlines. And I have met hundreds of entrepreneurs living in cities in “flyover country” that are building great companies and creating jobs in a wide range of industries.
back to top 

A drug used to treat advanced breast cancer has had what appears to be unprecedented success in prolonging lives in a clinical trial, researchers reported on Sunday.
Patients who received the drug — Perjeta, from the Swiss drug maker Roche — had a median survival time nearly 16 months longer than those in the control group.
back to top 

Medimmune, the global biologics research and development arm of Anglo-Swedish drug major AstraZeneca (LSE: AZN), has entered into a collaboration to establish a joint lab in Cambridge, UK, with Cancer Research Technology, the commercial arm of Cancer Research UK.
The new laboratory will be the first partnership of its kind of both organizations, and will focus on the discovery and development of biologic cancer treatments over an initial five-year period.
back to top 

Officials from local and state government as well as business and education attended a virtual ribbon cutting on Wednesday to celebrate the opening of the new $71 million Center for Communications and Information Technology at Maryland’s Frostburg State University.
“This is one of the most technologically advanced learning centers in the United States. Every inch of this building fosters learning,” said Jonathan Gibralter, FSU president.
back to top 

The Baltimore Business Journal has selected honorees for its first-ever Health Care Innovators awards.
These winners created new health care products and strategies that have made health care more accessible, efficient and effective. They will be featured in the Nov. 7 issue of the Baltimore Business Journal and will be recognized at a breakfast at the Hotel at Arundel Preserve on Nov. 7. Farzad Mostashari, the former national coordinator for health IT for the federal health department, will be the keynote speaker at the event.
back to top 

The FLC is pleased to offer its latest on-demand, and FREE, e-learning course, “Introduction to CRADAs”! If you’re new to tech transfer and need to know more about one of its most important mechanisms, this introductory-level e-course is perfect for you.
Available free of charge and at your convenience, the course covers essential CRADA knowledge:
- CRADA function and purpose
- What CRADAs can accomplish
- When CRADAs are used.
back to top 

GSK and venture capitalists Avalon have launched two early-stage R&D biotechs in San Diego, California.
Silarus Therapeutics and Thyritope Biosciences will each receive $10m (€8m) in a Series A financing round to investigate the hormone behind anaemia, and anautoimmune disease.
back to top 

With so much venture capital being foisted onto the digital health space, it’s beginning to beg the question: how long will this last, can it sustain itself, and what’s an entrepreneur to do? And, what are the implications for emerging companies versus traditional healthcare companies and systems?
Those were just a few of the burning questions discussed at Health 2.0‘s Pre, Post, M&A IPO panel held in Santa Clara.
back to top 

American University’s Kogod School of Business on Friday plans to show off its new on-campus start-up incubator, the latest in a string of co-working spaces to pop up in and around the nation’s capital.
The incubator, one of the key components of the school’s recently announced Sustainable Entrepreneurship and Innovation Initiative, aims to provide current students and recent graduates with work space and pair them with a business mentor to help get their fledgling ventures off the ground. In addition, each business team will received a $1,500 grant to cover some initial start-up costs, such as the legal work necessary to incorporate a company.
back to top 
New Enterprise Associates, a venture capital firm that has backed the likes of Groupon and Salesforce, is now investing in a biotechnology company developing a treatment for cancer.
The venture firm, which has a significant health care business, has led a $104 million financing round in Adaptimmune Limited, the company announced on Wednesday evening. The round, at the Series A stage, is believed to be among the largest for a biotechnology company at this early point of its development.
back to top 

The Atlantic Vaccines and Immunotherapeutics Summit is a first-of-its-kind event to showcase Maryland’s global leadership as an epicenter of vaccine innovation, development and commercialization. The Tech Council of Maryland is presenting this conference to bring together the industry’s foremost researchers and business leaders for the purpose of educating, sharing, and collaborating on important issues affecting the new generation of vaccines.
The Summit’s agenda will focus on a spectrum of topics including global R&D; government priorities and challenges; regulatory processes and policies; university and academic development; models for regional synergy; and vaccine market impact. We invite you to join us for this two-day event on May 7-8, 2015 at the Bethesda North Marriott in Bethesda, Maryland.
back to top 

Winners of the second annual Baltimore Innovation Awards included a civic hacker and a youth outreach organization.
The awards, a centerpiece of Baltimore Innovation Week’s Innovation Celebration, held Friday outside Under Armour headquarters in Tide Point, were announced in a brief presentation by Christopher Wink, editorial director and cofounder of Technical.l
back to top 

The success of Alibaba’s blockbuster initial public offering in the U.S. has catapulted founder Jack Ma into the limelight and given him the status of China’s richest man.
The making of Alibaba began in 1999 when e-commerce was unheard of in China. Recalling the days when he started the venture with 17 friends in his flat in the South-eastern Chinese city of Hangzhou, Ma once said: “I called myself a blind man riding on the back of blind tigers.” 15 years later, the firm is the dominant player in China’s e-commerce space. Between its two main online marketplaces Taobao and TMall, Alibaba accounts for nearly 80 percent of the mainland’s e-retail transactions.
back to top 

Tanisha Robinson is on tech startup No. 4 or so, with two running simultaneously at the moment, and aiming to make the latest a billion-dollar business. Here’s her best test to find if someone has what it takes to become an entrepreneur:
“If I were to take your wallet and your phone and your keys, and say you have to survive on your wits for a week,” she said.
back to top 

Being an entrepreneur can mean a demanding, unpredictable schedule; spreading oneself way too thin; and trying to pull off tremendous, seemingly impossible feats. This sometimes leads to burnout, and even if we don’t want to admit it, unhappiness. Matthew Toren penned a piece for Entrepreneur about habits of healthy, happy, and wise entrepreneurs. One of the best practices that leads to happiness? Setting and enforcing boundaries. Sounds obvious, but definitely easier said than done when you’re trying to please everyone from employees to spouses. Toren recommends:
back to top 

In the 24 years since the founding of the Georgia Research Alliance, federally-funded research and development grants to Georgia’s universities has increased five-fold.
The state’s total share of federal research funding increased to nearly 3 percent, ranking 12th and one of only five of the top 16 states that is increasing its market share.
back to top 

The Texas Medical Center has more square footage, more doctors and more hospitals than any other medical center in the country. However, that has not translated into product commercialization necessarily.
Its new accelerator may be the catalyst that changes that, experts say.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 7
Bethesda Country Club

October 7
Johns Hopkins University Montgomery County Campus

October 9

October 9
Microsoft Corporation

October 15
Johns Hopkins University – Montgomery County
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

 Tasly Pharmaceuticals, Inc., a global leader in pharmaceuticals, biologics, and nutraceuticals, held a ribbon-cutting ceremony on September 3, 2014, at Tasly’s corporate offices in Rockville, MD. Tasly Pharmaceuticals, Inc., a global leader in pharmaceuticals, biologics, and nutraceuticals, held a ribbon-cutting ceremony on September 3, 2014, at Tasly’s corporate offices in Rockville, MD.
The ceremony marked the official launch of Tasly’s Rockville location as the North American headquarters of Tasly Holding Group, a twenty year old global technology company established in China.
More than 100 distinguished guests and dignitaries were in attendance, including the Chairman and the President of Tasly Holding Group, China’s Embassy Counselor, Maryland state officials, Maryland biotechnology and pharmaceutical executives, and high-level regional media representatives, among others.
back to top 

This editor attended TEDMED 2014 in DC and had a chance to speak with some of the innovative start ups in the Hive, a showcase of new medical technologies.
BeneVir has developed an impressive immunotherapy for cancer that has the potential to become a lasting, durable cure to the disease. While other existing immunotherapy treatments exist, these solutions only help the immune system scour the body for known cancer cells afflicting a patient. These solutions fail to educate the immune system on how to predict, recognize, and kill cancer mutations that avoid the immune system and lead to a recurrence of the disease. BeneVir’s solution uses a natural human virus whose genes have been altered to allow the virus to target both the original cancer cells and their mutations. Prior to becoming a company, BeneVir’s founders originally developed their immunotherapy concept and licensed the solution to T-Vec, which was acquired by Amgen for $1B in 2011. This original concept recently completed a Phase 3 clinical trial showing a 16% durable cure for melanoma. With this success, BeneVir was formed to expand on the original innovation with what the founder calls version 2.0 plus. With deep technical knowledge, BeneVir is refining their original immunotherapy solution to become an even more potent cancer therapy.
back to top 

OpGen, Inc., a whole-genome analysis company developing and commercializing a complete suite of break-through products and services based on its proprietary Whole Genome Mapping technology, announced today the appointment of Vadim Sapiro as chief information officer. In addition, the company announced the promotion and appointments of several key members of the executive committee. The organizational changes have been made to strengthen the management team and accelerate OpGen’s development and commercialization strategy. The company’s products enable rapid, accurate, high resolution whole genome analysis of microbes as well as more cost effective and accurate sequence assembly and finishing of human, animal, plant and microbial genomes.
back to top 
 The 2014 NIH Research Festival, the annual showcase of the NIH Intramural Research Program, will be held Sept. 22-24. This year’s theme is “The Era of the Brain.” The festival kicks off with an opening plenary session at 10 a.m. on Monday, Sept. 22 in Masur Auditorium, Bldg. 10. The plenary session will include remarks by NIH director Dr. Francis Collins, followed by the FARE awards ceremony and scientific talks by Drs. Antonello Bonci (NIDA) and Mark Hallett (NINDS). The 2014 NIH Research Festival, the annual showcase of the NIH Intramural Research Program, will be held Sept. 22-24. This year’s theme is “The Era of the Brain.” The festival kicks off with an opening plenary session at 10 a.m. on Monday, Sept. 22 in Masur Auditorium, Bldg. 10. The plenary session will include remarks by NIH director Dr. Francis Collins, followed by the FARE awards ceremony and scientific talks by Drs. Antonello Bonci (NIDA) and Mark Hallett (NINDS).
back to top 

Oculus CEO Brendan Iribe first began working with computers in the 1980s, just as personal computers were entering homes. “I always said that it was the most exciting, amazing time,” he says. “We had no idea that in some number of years, a personal computer would within two physical taps, revolutionize, say, the entire taxi service industry.”
He has since revised his definition of the most exciting time to be in computer science to “now.”
back to top 
 Wednesday, October 15, 2014, 08:30am – 11:45am Wednesday, October 15, 2014, 08:30am – 11:45am
Johns Hopkins University – Montgomery County
This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.
Contact Ethan Byler at ebyler@biohealthinnovation.org if you are interested in scheduling one-on-one meetings.
More Information
back to top 

The 2014 Innovation2Commercialization Conference will be held on October 23rd at the Universities at Shady Grove in Rockville. This is our third year and we would like to ask you participate again to support and encourage the innovators, scientists, technologists and entrepreneurs in our area.
The conference will be held from 7:30am-3:30pm, with three panels: Innovation, Commercialization and Financing, and a special Keynote Speaker – Rachel King, CEO, GlycoMimetics. The program will spotlight serial entrepreneurs and successful tech transfer to product from the Naval Research Laboratory and The Johns Hopkins University – including a full panel discussion on the successful development to exit for Amplimmune.
As Partners in the past, we hope you found participation in the I2C Conference productive and plan to join us again this year.
back to top 

AstraZeneca and Eli Lilly and Company have reached an agreement for the development and commercialization of the oral beta secretes cleaving enzyme (BACE) inhibitor, AZD3293, which might become a viable treatment for Alzheimer’s disease. The oral therapy is expected to prevent the formation of amyloid plaque, which is comprised of peptides called amyloid beta, and slow the disease progression.
The agreement established stipulates that AstraZeneca will pay up to $500 million in development and regulatory milestone payments. Lilly announced that it is planning on receiving the first milestone payment of $50 million in the first half of 2015. Future costs will be equally shared by both companies, including net global revenues post-launch.
back to top 

Tuesday, October 7, 2014 from 6:00 PM to 8:00 PM (EDT)
Rockville, Maryland
In February 2013, The Sunshine Act was included as the Transparency Reports and Reporting of Physician Ownership or Investment Interests section of the Patient Protection and Affordable Care Act (ACA). The Sunshine Act requires manufacturers of drugs, medical devices, and biologicals that participate in U.S. federal healthcare programs to report certain payments and items of value (typically $10 or more and totaling $100 annually or greater) given to physicians and teaching hospitals. Failure to stay in compliance may result in fines ranging from $10,000 to $1,000,000 annually. Whether you are a practicing physician or your startup has a medical device, drug, or related product, you are impacted by the ACA’s Physician Payments Sunshine Act. In July, CMS proposed removing the reporting exemption for any payments or transfers of value made to physicians who participate in accredited CME programs. We’ll talk with experts in compliance and policy who will share their experiences in implementing these new policies and how you can understand the implications of the law, dispute inaccuracies, and stay in compliance!
back to top 

The Johns Hopkins University in Baltimore and other research institutions closer to Greater Washington have received hundreds of thousands of dollars from the ALS Association in the past few years.
Early indications are that some are likely to see even more in the future as contributions to the viral “Ice Bucket Challenge” have surpassed $113 million.
back to top 

Accepting Applications for InvestMaryland Challenge!
Could $100,000 help jumpstart your startup? Well, Maryland’s national business competition is back for the third year!
Since 2013, InvestMaryland Challenge has awarded nearly $1.5M in prizes to innovative startup companies. Prizes have included cash grants, incubator space, marketing and legal services, and opportunities to pitch investors.
Start an application today and your business could be on its way to accepting the $100,000 Grand Prize!
back to top 

An entrepreneurial and innovative spirit filled the conference room Tuesday, Sept. 16, at the Talbot County Free Library in Easton.
As part of Innovation Week, the third annual Startup Maryland Pitch Bus tour made a stop at the library. Business representatives from the additive manufacturing industry, commonly known as 3-D printing, also had 3-D printers and products on display.
The pitch bus offered an opportunity for area entrepreneurs to make a video for public viewing on YouTube to compete for venture investment.
back to top 

A competition by Washington, D.C.-based incubator 1776 in a global search for the finest startup has set down the groundwork for its annual Challenge Cup in search of technology and healthcare startups. It is working with incubators in 16 cities around the world to identify early-stage companies across education, energy health and environment in a new twist to the competition called ChallengeX.
It begins in Washington, D.C. on October 21 and in Chicago on October 28, according to a company statement. In addition to those cities, it is working with incubators in Sydney, New York City, Tel Aviv, Amman, Santiago, Nairobi, Mumbai, Austin, Toronto, Boston, Berlin, Dublin, San Francisco and China. Each city will have four winners in each of the categories and advance to a week long festival in May when entrepreneurs for these companies have a chance to pitch investors, receive mentoring and meet policymakers. The intention of the Challenge Cup is to search for meaningful technology that’s also scalable.
back to top 

More than 300 entrepreneurs are expected to vie for between 150 and 175 spots on the Startup Maryland bus to film a pitch video of their idea.
Now in its third year, the Startup Maryland’s Pitch Across Maryland tour will make 27 stops where entrepreneurs can film a short pitch video about their business. A panel of judges selects eight finalists, of which a winner and runner up are entered into a finalist round of the state’s InvestMaryland Challenge business competition.
back to top 

DC I-Corps, a new, NSF-supported program designed to foster, grow and nurture an innovation ecosystem in the Mid-Atlantic Region, is now accepting applications for its fall cohort, beginning on October 9. Applications will be accepted on a rolling basis up until that date. Apply here.
Open to research teams and technology entrepreneurs from universities, federal laboratories, agencies and the general community, the free program guides researchers in exploring the commercial potential of their inventions.
back to top 
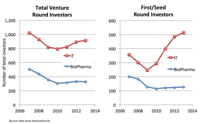
The ever-shrinking number of biotech venture capital firms was a common refrain during the 2008-2012 period; it’s true that a large number of firms went under, closed their doors for new investments, or moved into zombie status. I wrote on the subject back in July 2012 (here). The biotech investing environment then, with a relatively bleak IPO landscape and venture funding off some 40% from the prior year, was very different from the past two years with an “open” IPO window and steady pace of venture funding.
back to top 

On September 16, the American Academy of Arts and Sciences released a report entitled Restoring the Foundation: The Vital Role of Research in Preserving the American Dream. The report addresses the link between scientific research and advancing innovation and prosperity, and contains recommendations for strengthening its role to address challenges in the 21st Century. The recommendations fall under three overarching objectives: securing America’s leadership in science and engineering research; ensuring that the American people receive the maximum benefit from federal investments in research; and regaining America’s standing as an innovation leader by establishing a more robust national government-university-industry research partnership.
back to top 

As of 2014, Deloitte is the largest professional services firm in the world by revenue. What began in the 19th century as stand-alone accounting offices has merged and evolved into a partnership that encompasses 200,000 employees in more than 150 countries around the globe.
But size alone doesn’t make a company great, of course. Things like satisfying clients, increasing opportunities for personnel, and performing a vital function for society make a company great. When employees, customers, investors, and the community benefit from a company’s services, that’s what makes it stand out from the rest.
back to top 

Washington state’s Providence Health & Services today said it is launching a $150 million venture capital fund to help spur innovation across the healthcare system that lowers costs and leads to better outcomes.
Coincidentally, it’s the second VC fund announced in as many days on the West Coast, following yesterday’s announcement from the University of California.
back to top 

FasterCures, part of the Milken Institute dedicated to improving drug development by encouraging greater patient involvement is searching for digital health entrepreneurs to pitch at its upcoming conference, Partnering for Cures. The group is looking for collaboration opportunities between biotech and digital health, partly to find more effective ways to improve clinical trial recruitment.
Entrepreneurs for Cures will spotlight entrepreneurs who are developing digital and mobile health technology that boost patient participation in biomedical research and development and those looking for partnerships with patient communities to demonstrate their product’s capabilities. It’s particularly interested in companies pursuing solutions geared to chronic conditions, acute and infectious diseases, mental illness, genetic conditions.
back to top 

Since its passage more than two years ago, the Jumpstart Our Business Startups (JOBS) Act, which was designed to decrease regulatory burdens for raising capital, has spurred more than 110 IPOs among biotechnology companies alone. These are companies working to find cures for cancer, cardiovascular disease, blood disorders, and infectious diseases, among other conditions. The Securities and Exchange Commission (SEC) is now in the process of finalizing another important provision of the law that could further encourage biotech capital formation and investment.
back to top 

Dutch venture capitalist Aglaia has announced the launch of Aglaia Oncology Fund II. Investors contribute a total of $65 million to the fund, whose target size is $80 to $100 million. Like its predecessor Aglaia Oncology Fund I, the fund is backed by high-net-worth families. The group of investors has now been expanded to include several institutional investors, including the European Investment Fund (EIF), which has made a substantial commitment. Aglaia is also one of the funds selected under the Dutch Venture Initiative, an investment fund recently launched by the Netherlands Ministry of Economic Affairs.
Through the new fund, Aglaia will be investing in an estimated ten to fifteen biotechnology start-ups which are in the process of developing groundbreaking technologies aimed at preventing and curing cancer.
back to top 

The intersection of health and big data is getting more crowded. San Mateo, Calif.-based Lumiata is the latest company to get venture backing for technology that crunches data with the aim of improving medical care, Yuliya Chernova reports for Dow Jones VentureWire.
The company has already run an analysis of some 100,000 BlueCross BlueShield patients that found about 10,000 people who were very likely to have congestive heart failure, even though they were undiagnosed. Now the insurance provider, which is also an investor in the company, is alerting doctors who have those patients about this potential missed diagnosis.
back to top 

AS a former physician, I shivered a bit when I heard Dr. Vivek Wadhwa say he would rather have an artificial-intelligence doctor than a human one. “I would trust an A.I. over a doctor any day,” he proclaimed at a recent health innovation conference in San Francisco, noting that artificial intelligence provided “perfect knowledge.” When asked to vote, probably a third of those in attendance agreed.
But it made sense: Dr. Wadhwa is a professor, entrepreneur and technology visionary. What’s more, the conference took place in San Francisco, where faith in the power of technology and data to solve problems holds unshakable sway.
back to top 

Recognizing the continuing evolution of New Jersey’s life sciences industry, BioNJ today released a comprehensive report that documents the ongoing growth of the biotechnology sector in the State and combines and assesses the contributions of the entirety of the life sciences sector to New Jersey’s economy, including employment and economic impact.
BioNJ conducted the study in partnership with EY (formerly known as Ernst & Young) the Edward J. Bloustein School of Planning and Public Policy (Bloustein School) and the New Jersey Department of Labor and Workforce Development’s (LWD) Office of Research and Information. To access the study go to: http://www.bionj.org/wp- content/uploads/2014/09/Industry-Study-9-19-14-Final-Final.pdf.
back to top 
Earlier this year, both Apple and Google presented competing visions for how we’ll use apps and wearables to gather data about our bodies and share it via our phones. Apple called its software HealthKit, while Google presented Google Fit.
If things had gone according to plan on Wednesday, Apple would be enjoying a head start over Google, with HealthKit released along with iOS 8, its new mobile software for iPhones and iPads. (Google Fit is still unreleased beyond a “developer preview.”)
back to top 

Some of the trends in American health care are obvious: Managing costs in the age of Obamacare, patients using online information to take charge of their own health and wellness, and finding ways to deliver care in settings other than hospitals and clinics.
Those trends become specific, sometimes knotty, challenges for people engaged in building the tools needed to effectively, efficiently and safely deliver health care.
Envisioning, designing and manufacturing medical devices – which range from robots to sensors, and from surgical instruments to software that allows devices to communicate with one another – was the topic of a conference Thursday and Friday in Chicago.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 22-24
NIH Building 10

September 25
MedImmune

October 7
Bethesda Country Club

October 7
Johns Hopkins University Montgomery County Campus

October 9
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI) announced today that Roche Pharma Research and Early Development (pRED) and BHI have entered into an agreement to advance healthcare technologies coming from academic institutions, federal laboratories and startups based in Central Maryland. Under the terms of the agreement, BHI will identify health technologies that Roche will evaluate for potential research, development and commercialization opportunities. Priority areas of interest will include oncology, neuroscience, ophthalmology, rare diseases, immunological and infectious diseases. Financial terms for the agreement were not disclosed.
The agreement was initiated through Roche pRED Academic Relations and Collaboration (ARC) group, which is led globally by Juan Carlos Lopez. “Sourcing of technologies from academia, federal labs and early-stage startups can be a challenging and lengthy process,” said Lopez. “As we seek to expand our capabilities to source and screen technologies aligned with our strategic interests, BHI is an ideal partner to support identification of external opportunities in a broad, systematic fashion.”
back to top 
 Tasly Pharmaceuticals, Inc (Tasly) and BioHealth Innovation (BHI) co-organized the first Tasly Meeting of CEOs on Tuesday, September 2, 2014 at the Tasly facility in Rockville. Attendees included more than 25 CEOs and company representatives from local Maryland-based BioTech and BioPharma companies. This was the first public event conducted through Tasly and BHI’s newly initiated partnership. Tasly Pharmaceuticals, Inc (Tasly) and BioHealth Innovation (BHI) co-organized the first Tasly Meeting of CEOs on Tuesday, September 2, 2014 at the Tasly facility in Rockville. Attendees included more than 25 CEOs and company representatives from local Maryland-based BioTech and BioPharma companies. This was the first public event conducted through Tasly and BHI’s newly initiated partnership.
Tasly is an innovation-based, high-tech healthcare and pharmaceutical enterprise and world-leader in the research and product development (R&D) of Chinese herbal medicine – the leader in the natural way to modernize medicine, with a North American headquarters in Rockville, Maryland, . Tasly and BHI have a formalized partnership which aims to co- organize and coordinate joint BioHealth associated events to promote professional networking, business growth, and technology and product commercialization.
back to top 
 Wednesday, October 15, 2014, 08:30am – 11:45am Wednesday, October 15, 2014, 08:30am – 11:45am
Johns Hopkins University – Montgomery County
This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.
Contact Ethan Byler at ebyler@biohealthinnovation.org if you are interested in scheduling one-on-one meetings.
More Information
back to top 

Sebastian Seiguer had already founded emocha Mobile Health by the time his company began its four-month term in January 2014 as part of DreamIt Health Baltimore, an accelerator for developing health IT and health care startups that graduated its first class in May. But Seiguer’s venture, an online remote patient management platform for clinicians to monitor patients’ symptoms and recovery via smartphone, was a one-man operation, and he needed help.
“The rallying point was the accelerator,” says Seiguer, who launched emocha in fall 2013 by licensing technology from the Johns Hopkins University. “It allowed me to quickly recruit a strong team because there’s a lot of excitement generated by joining an accelerator and a solid program.”
back to top 

Please plan to attend and also help spread the word about some exciting technology transfer programs planned for the NIH Research Festival next week:
Monday, September 22nd from 12-2pm
“Careers for scientists in technology transfer & business development” Poster Session I Building 10 – FAES Academic Center Presenter: Steve Ferguson (NIH/OTT)
Monday, September 22nd from 12-2pm
“NIH and the Federal Laboratory Consortium for Technology Transfer” Poster Session I Building 10 – FAES Academic Center Presenters: Steve Ferguson (NIH/OTT) and Gary Jones (Federal Laboratory Consortium)
Monday, September 22nd from 2-4pm
“Commercial Development of my Research: Still More Personal Stories from former NIH Intramural Scientists” Concurrent Symposium Session I Building 10 – FAES Academic Center – Classroom 3
Co-chairs: Steve Ferguson (NIH/OTT) and Todd Chappell (BioHealth Innovation)
The full schedule of events at the NIH Research Festival (September 22-24) can be found at: http://researchfestival.nih.gov/2014/index.shtml
back to top 

The CEO and co-founder of Oculus VR is giving the University of Maryland $31 million – the largest gift in university history – to construct a computer science building.
University officials said late Thursday that alumnus Brendan Iribe’s gift will help build the Brendan Iribe Center for Computer Science and Innovation. Most of the gift will go toward the building, which will be designed for work in virtual reality, augmented reality, computer vision and robotics, and $1 million will establish the Brendan Iribe Scholarship in Computer Science.
back to top 

Harpoon Medical, Inc., a medical device company focused on the development and commercialization of a minimally invasive, beating heart mitral valve repair technology, announced today that the company has successfully raised $3.2M of new capital and expects to complete the round over the coming weeks. The Series A financing was led by Epidarex Capital in order to advance Harpoon’s innovative technology into the clinic and expand the company’s research and development efforts.
“We are very pleased with the results of our Series A financing because it gives Harpoon Medical the resources necessary to advance the technology from an innovative concept into the clinic,” said, Bill Niland, CEO of Harpoon Medical, Inc. and serial healthcare entrepreneur. The round was increased to accommodate strong demand from investors that include the Maryland Venture Fund, the Abell Foundation, medical device executives, doctors and successful business executives in the Maryland area. “When you have the opportunity to put together a high caliber syndicate of investors who can help you with more than just capital you find a way to accommodate the demand.”
back to top 

Brendan Iribe dropped out of the University of Maryland here, but before he did he amassed 227 parking tickets. And he managed to meet two business partners who would help him build the virtual-reality company Oculus VR, which Facebook bought this year for about $2-billion.
One of those parking tickets remains unpaid, but the university is likely to forgive it after Friday, when he gave $31-million to erect a computer-science building. That makes Mr. Iribe, who is 35 years old, the institution’s most generous donor ever.
back to top 

The Johns Hopkins University has entered into a partnership agreement with Google’s Advanced Technology and Projects group aimed at speeding up the development of new technology and moving the resulting products toward the marketplace more quickly.
The agreement will enable ATAP to draw on the expertise of computer scientists and others at Johns Hopkins and approve funds for joint technology projects in as few as 30 days. That turnaround time is much shorter than the period typically required for obtaining grants from government agencies and private organizations.
back to top 

As the Ebola death toll climbs above 2,000, a university researcher is working to develop a vaccine for the virus.
Alan L. Schmaljohn, a microbiology and immunology professor at University of Maryland, Baltimore, has successfully isolated one of the structural sections of the virus, which can be used as a platform for a working vaccine.
back to top 

Pluristem Therapeutics, a company that develops placenta-based cell therapy products, announced that its licensing partner United Therapeutics is advancing the Phase I study of Pluristem’s PLacental eXpanded (PLX-PAD) cells in patients with pulmonary arterial hypertension (PAH).
PLacental eXpanded (PLX) cells are developed by Pluristem to serve as protein delivery platforms that release a mix of proteins for ischemia or inflammation. The treatment technology is also being used to investigate tendon-healing treatments in a preclinical model of tendon injury. The company presented results at the American Academy of Orthopedic Surgeons’ (AAOS) Annual Meeting last March in New Orleans.
back to top 

In the fight against cancer, Johns Hopkins University is invoking a popular attitude these days: go big or go home.
The Johns Hopkins radiation oncology department has teamed up with Toshiba Group to establish the Toshiba Center for Big Data in Healthcare at the Science + Technology Park at the Hopkins campus in Baltimore.
back to top 

A white powdered chemical compound emerged from two University of Maryland School of Medicine laboratories more than 10 years ago with a name destined for oblivion, but a future that now looks promising as a treatment for the most challenging cases of prostate cancer.
Today, VN/124-1 is a drug candidate with a name — galeterone — a pharmaceutical company founded on its potential and a record of strong preliminary results in clinical trials with human patients.
back to top 

Startup Maryland announced support from TEDCO (www.tedco.md) as a full-tour sponsor of the Pitch Across Maryland 2014, the third annual state-wide tour and celebration of entrepreneurship and startup companies.
Starting on September 15 and running through October 3 the Pitch Across Maryland tour will again traverse the state all in the name of celebrating Maryland’s diverse communities of venture building.
“Maryland’s innovation economy is front-and-center in many of the most lucrative industry and innovation categories,” stated Michael Binko, founder of Startup Maryland. “From traditional ‘feds, eds and meds’ (government/education/healthcare/life sciences-bio), to cloud computing, cyber-security, creative/arts, clean energy/green, to autonomous systems, Maryland entrepreneurs are breeding innovation ventures that are disrupting a wide array of industry sectors and TEDCO has consistently been the recognized leader in early-stage resource commitments.”
back to top 

Optum Labs has named the University of Maryland, Baltimore (UMB) as one of the latest partners to join its research collaborative. Led by Eleanor Perfetto, PhD, MS, professor in the Department of Pharmaceutical Health Services Research (PHSR) at the University of Maryland School of Pharmacy, this new partnership will enhance and augment UMB’s existing research and informatics resources with the data, tools, expertise, and infrastructure available at Optum Labs to increase the scope and impact of Alzheimer’s disease and healthy aging research.
“This partnership with Optum Labs enhances UMB’s recognition as a leader in ‘big data’ research,” says Perfetto. “In addition to expanding research opportunities for faculty and students across the University, the partnership increases our competitiveness for grants and contracts from industry, government, and philanthropic organizations. We look forward to combining our expertise and resources with those at Optum Labs to pursue innovative projects that will improve health care delivery and patient outcomes for individuals with Alzheimer’s diseases and other aging-related issues.”
back to top 

Some of the greatest health care innovations have taken decades to reach widespread adoption, adding to the ever-increasing cost of health care.
We are looking for solutions that have already been implemented at least once and have demonstrated effectiveness. This “scale-up” competition seeks to shorten the time frame for innovation dissemination.
back to top 

Say you invent a medical device. A pacemaker. An improved hip implant. A microchip for the brain. Maybe you can change the world, but first you’ll have to get approved.
A new graduate certificate program in the bioengineering department teaches students the ins and outs of gaining Food and Drug Administration approval. This process is necessary to test the safety and efficiency of all medical inventions before they hit the market, but it can take years of expensive research — and disapproval is common, said William Bentley, the bioengineering department chairman.
back to top 

When we go grocery shopping, most of us don’t buy a pallet of one product and survive on that for the rest of our lives. We get our food more regularly, so it’s fresher and more in tune with our tastes of the moment. So why wouldn’t education in the fast-changing STEM fields, look like that too?
That was how Andrew Coy, the executive director of the Digital Harbor Foundation, put it during an informal tech leaders roundtable discussion at City Hall Thursday morning.
back to top 

Today, the University announces the receipt of its largest gift ever by a single donor. It will catapult our top-15 computer science program to even greater national and international pre-eminence. It will spark innovation and entrepreneurship across the campus and catalyze new economic development in the state.
The gift began after a tragedy and will end in a living memorial. It demonstrates the impact of friendship, teamwork, and family—qualities that ultimately benefit our students and faculty.
back to top 

The innovation program designed to move academic findings and translational research into the commercial marketplace, known at Johns Hopkins University as FastForward, is expanding early next year, with a second facility scheduled to open in East Baltimore to provide lab and office space for startups. Some lab space will be available starting in September 2014.
The first accelerator, FastForward Homewood, at the historic Stieff Silver Building near Homewood campus, was opened by the Whiting School of Engineering in June 2013, and today it houses nine startups from across the university. The second incubator, FastForward East at the Rangos Building on North Wolfe Street, will be more closely tied to the Johns Hopkins School of Medicine, the Bloomberg School of Public Health, and the Johns Hopkins School of Nursing, while still allowing scientists and technical experts from a wide background to take advantage of the new facility.
back to top 

Get answers now from experienced entrepreneurs and legal/business professionals on how to build a successful startup company. Receive free and impartial advice, brainstorm business strategies, investigate funding opportunities and learn about the vast resources available to entrepreneurs.
DATE: September 16, 2014
PLACE: Columbus Center, 701 East Pratt Street, Baltimore, Maryland 21202 USA
REGISTER: Schedule an appointment here to guarantee your time slot. Walk-in appointments are also available.
back to top 

There were few surprises for local colleges and universities in the oft-quoted U.S. News and World Report annual rankings released Tuesday.
The Johns Hopkins University maintained its 12th-place position for national universities, falling between Dartmouth and Northwestern. Hopkins is focusing on its undergraduate experience, with a goal of making it among the top 10 in the nation by 2020.
back to top 

nvestors in entrepreneurs have a special in-the-trenches wisdom gained through years of experience. This investor experience is now available to entrepreneurs and prospective early-stage investors through InvestorIQ.org, a free, online curriculum that provides knowledge essential to improving startup investment.
Investor IQ allows startup backers and founders to educate themselves on how investors decide whether to invest at all, where to invest, and how their money will be used and returned. A video series draws on the successes and failures of thousands of angel investors over the last 15 years, delving into five questions early-stage investors should ask themselves before they put money into an entrepreneurial venture.
back to top 

It’s no surprise that social-media campaigns can raise awareness of an issue, but the ALS Ice Bucket Challenge may be unprecedented in its impact on a relatively rare disease. The campaign, in which participants must donate to an ALS cause or take videos of themselves being doused in ice, has gone viral since it began in late June. But it has also generated controversy, with some questioning the attention and flood of cash for a disease that affects a small number of people.
As of Friday, the ALS Association had received $53.3 million since July 29, compared with $2.2 million by that time last year. To put it in perspective, the National Institutes of Health’s yearly budget for ALS research is $40 million. Other ALS charities are also benefitting similarly from the campaign.
back to top 

The Southern Maryland Innovation and Technology (SMIT) initiative has awarded Smartronix and Coherent Technical Services, two locally headquartered technology firms, Pro Memberships to CoFounders Lab.
CoFounders Lab, a OneVest Company, is an online partnering platform of more than 40,000 founders, advisers and interns available to help launch and grow businesses. A Pro Membership provides access to entrepreneurs nation-wide to help individuals connect with the right partner, investment or resource needed to take a business to the next level. SMIT will award 40 Pro Memberships to individuals and companies to encourage technical innovation and commercialization in St. Mary’s County.
back to top 

Over the course of three days at the Mayo Clinic‘s annual Transform conference, featuring scores of healthcare’s top names, several themes stood out. Here’s what I gather were the main points.
1. Economic factors and barriers are just as important in determining health outcomes for the population as a whole. It stands to reason – people with fewer financial resources are often forced to choose between paying for a costly lab test or doctor visit or putting food on the table, among countless other instances and examples.
back to top 

My last post, How Successful People Stay Calm, really struck a nerve (it’s already approaching 1.5 million reads here on LinkedIn). The trick is that managing your emotions is as much about what you won’t do as it is about what you will do.
TalentSmart has tested more than a million people and found that the upper echelons of top performance are filled with people who are high in emotional intelligence (90% of top performers, to be exact). So, I went back to the data to uncover the kinds of things that emotionally intelligent people are careful to avoid in order to keep themselves calm, content, and in control. They consciously avoid these behaviors because they are tempting and easy to fall into if one isn’t careful.
back to top 

There has been a long-running debate in the pharmaceutical industry about the value of being first to market. Companies spend considerable resources seeking to increase the odds of beating their competitors to market and often fret about the commercial disadvantage of being late. In the high-stakes race to market for a novel drug class, companies firmly believe that every month of lead time ahead of a competitor is significant.
It’s not quite that simple. Our analysis of pharma launches confirms a weak first-to-market advantage on average, but with significant nuances dependent on market context. In many instances, the first-mover advantage actually vanishes, particularly when the lead time is short or when the first mover is a small company. This article seeks to identify those situations where first-to-market advantage is strong and those where it does not hold.
back to top 

This is the first in an exciting series of programs designed to keep you current on issues affecting you as a leader of your organization, and provides an opportunity to connect with your colleagues.
On October 7, Gary will discuss the role of disruptive technology in today’s market and how to create an adaptive, decisive, mission-oriented corporate environment that can help you drive your company forward. In addition to leading one of the largest associations of technology companies, Mr. Shapiro is at the helm of the International CES (Consumer Electronics Show), the world’s largest annual innovation event. It unites more than 150,000 retail buyers, distributors, manufacturers, market analysts, importers, exporters, and press from 150 countries.
Join us as Gary shares his passion for innovation and explains what it is like to be at the forefront of a multi-billion dollar industry and what is coming next.
I look forward to seeing you at this terrific event. Click here to register now!
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 15
Deadline

September 15-16
Sheraton Pentagon City

September 15
Various Locations

September 16
UMBC

September 18-19
Baltimore Marriott Inner Harbor at Camden Yards
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Emergent BioSolutions Inc. (EBS) announced today that it has signed a contract with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), to develop a dry formulation of NuThraxTM (Anthrax Vaccine Adsorbed with CPG 7909 Adjuvant), also known as AV7909, the companys next generation anthrax vaccine candidate. This five-year contract, valued at up to $29 million, provides funding for manufacturing and non-clinical activities through the preparation of an Investigational New Drug application to the U.S. Food and Drug Administration. The dry formulation of NuThrax is intended to increase stability of the vaccine candidate at ambient and higher temperatures, with the objective of eliminating the need for cold chain during shipping and storage.
back to top 

MIMETAS, in a consortium with Radboudumc and FHNW, has received 1.6 million USD funding for development of a kidney-on-a-chip for toxicological applications. A panel of experts from GlaxoSmithKline, Pfizer, Roche, NC3Rs and renowned academic institutions selected MIMETAS’ solution from a strong line-up of competing technologies.
The funding is awarded in the context of the NephroTube Crack-it Challenge to support development of a microfluidic renal model predicting renal toxicity during pre-clinical development. In collaboration with the Radboudumc Pharmacology-Toxicology Department and the Swiss FHNW, MIMETAS will use the funds to develop a high-throughput kidney-on-a-chip model by combining its OrganoPlate™ 3D-culturing technology with the human renal cell line ciPTEC™, analyzed and validated in three separate sites. The resulting model will be used to detect renal tubular injury observed in drug-induced nephrotoxicity. The model’s early prediction of nephrotoxicity will help to reduce animal experiments.
back to top 

The National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) in Bethesda, Maryland is seeking a dynamic, innovative and accomplished biomedical/biotechnology executive with demonstrated scientific and entrepreneurial expertise to provide strategic vision and leadership for the Office of Translational Alliances and Coordination (OTAC). The OTAC is charged with developing, implementing and leading translational research programs that create recognizable commercial value for discoveries and innovations during their gestational stages and facilitating their ultimate translation into new diagnostics, devices, therapeutics and tools. The Office is also charged with identifying emerging areas of translational opportunities, serving as a focal point for extramural researchers for information on NHLBI-wide small business technology development opportunities.
The OTAC Director is an expert in entrepreneurism and technology advancement with the vision and unique skill set required to recognize commercial value in very early stage technologies, guide their scientific and business oriented risk-mitigating development and the capital acquisition activities required for their ultimate customer/patient deployment. These skills, gained through experience in both the academic research and the start-up/small business or industry sector, will provide the OTAC Director the capability to oversee and effectively manage existing NHLBI translational research technology development programs and the development of new programs and initiatives required to identify and advance innovations and technology platforms for research, diagnosis, treatment, control and prevention of cardiovascular, lung, blood and sleep disorders.
back to top 

WHEN: Thursday, Sept 25th, 2014 From 3:00 to 5:00 PM
WHERE: One MedImmune Way Gaithersburg MD 20878
BioBuzz Double Helix Sponsor MedImmune is hosting a special BioNetworking event for Post-Docs and Professional Scientists on September 25th at their headquarters in Gaithersburg.
This event will include music, lawn games, hors d’oeuvres, drinks and a scientific “Speed Networking” session and is open to Post-Docs & Professional Scientists who currently work in the Therapeutic Areas of:
* Oncology
* Respiratory, Inflammation & Autoimmunity
* Cardiovascular & Metabolic Diseases
* Infectious Diseases & Vaccines
* Related scientific disciplines
To RSVP: email info to MedIPartneringStrategy@MedImmune.com Sign up by 9/12 and Provide your:
Name
Company
Phone
Therapeutic Area
Let them know you learned about this event through BioBuzz!
This event is for Post-Docs & Professional Scientists
back to top 

The I2C Conference is a full-day event to help you get your technology-based small business on the fast-track! Three in-depth panels on Innovation, Commercialization and Financing Speakers include: GlycoMimetics, MedImmune, Johns Hopkins University, Naval Research Labs, Brain Sentry, Mindoula, SmartSenseCom, TEDCO, Maryland Venture Fund, and the exit strategy team from Amplimmune. Enjoy lunch…
back to top 

Are you an entrepreneur ready to pitch your company? Register for your chance to film a three-minute video pitch to be seen online by VCs and angel investors and be voted on by fans around Maryland!
The Montgomery County Department of Economic Development and the Montgomery County Chamber of Commerce have partnered to host Montgomery County’s Pitch Across Maryland stops on October 1, 2014. Applications for the Montgomery County Pitch stops are due September 15!
back to top 

Swiss drugmaker Roche Holding AG said on Monday the European Union has approved the use of its drug RoActemra in patients with early-stage rheumatoid arthritis.
Roche said the European Commission has backed RoActemra as a treatment for patients with severe, active and progressive rheumatoid arthritis who have previously not been treated with methotrexate.
back to top 

When British pharmaceutical giant GlaxoSmithKline announced in October 2012 that it planned to make detailed data from its clinical trials widely available to researchers outside its own walls, the scientific community was stunned. For a company that spends $6.5 billion a year on research and development, it was a sharp turn away from the system of data secrecy that had made it one of the world’s largest drug companies, with 2013 sales of $43.6 billion.
The announcement came a few months after the company pled guilty to misdemeanor charges in the U.S. that it had marketed drugs for unapproved uses, based on improperly reported clinical trial data, and failed to report safety data on another drug later shown to raise the risk of heart attacks. Given the timing, many wondered if GSK’s move was more about rehabilitating its image than embracing data transparency.
back to top 
The 2014 International Biotech Summit Committee invite BioHealth Innovation (BHI) and Montgomery County to attend the three co-located international conferences that will be held on Sep 9 -12 in Shenzhen, China:
- 2014 International Biotech Summit (September 10, 2014);
- The 9th International Conference on Genomics (ICG-9) (September 11th-12th, 2014);
- Shenzhen International Biotech & Health Industry Expo 2014 (SIBHIE, September 10th-12th, 2014).
Ms. Lily Qi, Director of Special Projects for Montgomery County Executive Office who was involved in establishing BHI and a native of Shanghai, is going to speak on behalf of the County and BHI for the forum, “Innovation in Policy and Regulation for Advancing Healthcare Industry.” BHI and the County are also invited as an exhibitor for the SIBHIE Expo.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Implementation of the NIH Genomic Data Sharing Policy for NIH Grant Applications and Awards
- NIH Genomic Data Sharing Policy
- Public Comments on Proposed Guidance Regarding Significant Changes to Ongoing Animal Activities
- Guidance on Significant Changes to Animal Activities
- Notice of National Biosafety Stewardship Month and Health and Safety Requirements for NIH Grantees
- eRA Commons Username Required for Sponsor in Individual Fellowship Grant Applications to NIH and AHRQ
- NIAMS Policy for Submission of Applications Containing Clinical Trials
- (NOT-AR-14-021)
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
Requests for Applications:
- Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Centers (U54)
- (RFA-AR-15-002)
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
- National Heart, Lung, and Blood Institute
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Neurological Disorders and Stroke
- Application Receipt Date(s): November 28, 2014
back to top 

Maryland Industrial Partnerships (MIPS) Funding for Technology Product Development
MIPS provides funding, matched by participating companies, for university research projects that help companies develop new technology products.
Benefits to Maryland Companies
- Cost-effective research with world-class university faculty
- Access to university students, state-of-the-art facilities, laboratories and equipment
- Non-dilutive, non-debt funding for research
- Opportunity to work directly with talented students—potential future hires
- Rapid proposal evaluations—MIPS notifies award winners within 60 days of the proposal deadline
Benefits to Maryland Faculty
- Research translates directly to new product development
- Potential for published papers and improved university facilities
- Students gain valuable experience working on commercial technologies
Visit www.mips.umd.edu or call 301.405.3891301.405.3891 for details.
APPLICATIONS ARE DUE BY OCTOBER 15, 2014!
back to top 

SAVE THE DATE: THURSDAY, NOVEMBER 20, 2014
The MCCC Business Awards Dinner Committee and Chair of the Board Lisa Cines, CPA of Dixon Hughes Goodman LLP invite you to join in the celebration of those who make our economy and community thrive. This annual sold-out event attracts 700+ guests including award winners, sponsors, business leaders, elected and government officials and the media. Join us for a great evening of Meaningful Connections, Commerce and Celebration.
back to top 

A lot has been written lately about innovation, or the lack thereof, in the world of biopharma. One question that often gets asked: which countries lead the way in creating new medicines? Many people think that drugs originate in the nation where the companies that produce them are headquartered. The truth, however, is much more complicated. Given that multi-national firms market the majority of medicines, figuring out where each one of their drugs originated requires digging through some extensive data vaults. A proper analysis requires the examination of company histories, free market deal making, and in some cases government interventions. Consider the following examples:
back to top 

Have you always wondered what type of scientific researcher you are? Take the quiz to find out.
back to top 

This Monday September 1st marked the beginning of when investors with Angel Venture Forum D.C. started looking at submissions from companies for the Nov. Showcase.
AVF screeners do start reviewing plans as soon as submissions are made and some companies who have submitted to date have already received assistance and invitations to angel groups. Since we typically get a large number of applications and it does take the screeners a while to get through them all. Thus, the sooner an application the better.
The initial screening will occur through Sept. 15th. So you may still make submissions and corrections on applications up until Sept. 15th if needed. However, it is recommended that you complete your submission as soon as possible so that you get in the queue.
To submit an application – no later than Sept. 15th – visit www.angelventureforum.com. For any questions, please feel free to contact Mike Gildea, AVF COO at mgildea@braingainllc.com or 814-451-1151.
back to top 

The Food and Drug Administration on Thursday approved the first of an eagerly awaited new class of cancer drugs that unleashes the body’s immune system to fight tumors.
The drug, which Merck will sell under the name Keytruda, was approved for patients with advanced melanoma who have exhausted other therapies.
back to top 

GETTING IDEAS TO PATIENTS: THE SOPE FOUNDATION CROWDFUNDING CAMPAIGN NEEDS YOUR SUPPORT FOR EDUCATIONAL PROGRAMS
To provide SoPE members with educational programs, events and more, we need your support. The SoPE Foundation has started a crowdfunding* campaign —Getting Ideas to Patients—to raise $250,000 by the end of 2014 for educational programs and events. The SoPE Foundation is the sole funder for these activities, such as the Blakely Visiting Lectureship Series named posthumously for one of our co-founders, and support for biomedical and health innovators applying for the Innovation Scholars Program. SoPE’s operates on a low-cost membership model to encourage enrollment. With your financial contribution, we will have the funding to do what we set out to do. Every little bit helps, and every larger bit moves us closer to our goal.
back to top 

Dubbed as the most severe and deadly outbreak, the Ebola menace in West Africa has caught the attention of GlaxoSmithKline plc (ADR) (NYSE:GSK) and National institute of health. CNBC’s Meg Tirrell reports that NIH and GlaxoSmithKline have partnered to develop a vaccine that is said to have had immense success in primates, but yet to be tested in Humans.
The studies are to be used to ascertain whether the vaccine is safe and also its ability to prompt an immune response, able to combat the Ebola virus. No humans are to be infected’ with the Ebola virus during the course of the study.
back to top 

Two Washington entrepreneurs with a history of building successful companies have teamed with a local businessman to start a venture capital fund focused on seed stage companies focused on applications that run on mobile devices.
The fund, called Kiwi Venture Partners I , has raised $2.5 million from 24 limited partners, including 12 from the Washington area. The LP investments range from $25,000 to $500,000.
back to top 

According to CB Insights data, funding to digital health/health IT companies has already topped $2.2B in the first half of 2014.
With the space becoming increasingly crowded and well-funded, one effective way innovation trackers can understand which areas (and likely companies) will play a key role in future of the digital health space is by following the smart money.
back to top 

Anne Arundel Medical Center is looking to get in on technology commercialization, a game more typically played at research hospitals and universities.
The James and Sylvia Earl Simulation to Advance Innovation and Learning (SAIL) Center is developing a plan to bring together doctors and the local business community to commercialize technology that would benefit the hospital.
back to top 
Legal 500, considered one of the most comprehensive worldwide qualitative guides available on legal services providers, recently announced its 2014 rankings. Venable’s national Corporate Group continued to earn high marks in the latest edition leading the way with a Tier 1 ranking in the M&A: Middle-Market (Sub-$500m) category. The group also received high marks in the Technology: Outsourcing, Technology: Transactions and Real Estate Investment Trusts (REITs) categories.
Corporate Practice Co-Chair Charles J. Morton, Jr. and partner William T. Russell received special recognition as Leading Lawyers for M&A: Middle Market (Sub $500m) Technology: Transactions respectively. Leading Lawyers are selected from all the nationally recommended attorneys in a particular category and represent those few individuals, who, in the view of their peers, represent the highest standards of their practice. This is Mr. Morton’s third year as a Leading Lawyer and Mr. Russell’s fourth. Mr. Morton was one of 23 attorneys nationwide named a Leading Lawyer in M&A: Middle Market (Sub $500m). Mr. Russell was one of only 12 attorneys nationwide named a Leading Lawyer in Technology: Transactions.
back to top 

A group of UC Berkeley students launched what they call the first-ever student-run health tech incubator Thursday at the office of Downtown Berkeley’s Skydeck, which fosters campus startups.
The incubator, Catalyst@Berkeley, aims to provide a framework for students to bring viable prototypes to the market and open doors of entrepreneurship to undergraduate students interested in health care innovation.
back to top 

The Life Sciences Discovery Fund (LSDF) today announced nearly $750,000 in Proof of Concept grants to Washington state for-profit and non-profit organizations to promote translation of health-related technologies from the laboratory to the commercial marketplace. Also announced was nearly $56,000 in supplemental funding to an existing grant to increase the commercial potential of a drug to protect hearing in patients taking certain antibiotics. (See Backgrounder Information.)
The LSDF Board of Trustees selected the awardees following review of proposals for scientific and technical merit, commercial potential, and health and economic benefits to Washington.
back to top 

Friday, November 14, 2014
Encounter the hottest trends and innovations in Health IT as well as investment at the “eHealth Venture Summit”. This event at the MEDICA Health IT Forum showcases new companies, offers valuable insights and shall foster strategic partnerships and investment opportunities crucial to bringing innovative products to market. The event will feature seasoned industry experts from leading companies and funding specialists. The conference is jointly organized by Prof. Talya Miron-Shatz, CEO of CureMyWay, and a faculty member at the Ono Academic College, and Dr. Stefan Becker of the Institute for Drug Safety, University Hospital Essen.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9
UMD Mtech

September 10-12
Shenzhen Convention & Exhibition Center

September 10
Shenzhen Convention & Exhibition Center

September 10-12
Washington, DC | San Francisco, CA

September 10
Germantown Innovation Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
Legal 500, considered one of the most comprehensive worldwide qualitative guides available on legal services providers, recently announced its 2014 rankings. Venable’s national Corporate Group continued to earn high marks in the latest edition leading the way with a Tier 1 ranking in the M&A: Middle-Market (Sub-$500m) category. The group also received high marks in the Technology: Outsourcing, Technology: Transactions and Real Estate Investment Trusts (REITs) categories.
Corporate Practice Co-Chair Charles J. Morton, Jr. and partner William T. Russell received special recognition as Leading Lawyers for M&A: Middle Market (Sub $500m) Technology: Transactions respectively. Leading Lawyers are selected from all the nationally recommended attorneys in a particular category and represent those few individuals, who, in the view of their peers, represent the highest standards of their practice. This is Mr. Morton’s third year as a Leading Lawyer and Mr. Russell’s fourth. Mr. Morton was one of 23 attorneys nationwide named a Leading Lawyer in M&A: Middle Market (Sub $500m). Mr. Russell was one of only 12 attorneys nationwide named a Leading Lawyer in Technology: Transactions.
back to top 

GlaxoSmithKline (GSK) is one of the world’s leading research-based pharmaceutical and healthcare companies, has announced that it will freeze the prices of its vaccines for five years for developing countries that graduate from GAVI Alliance support.
By committing to offer GAVI Alliance prices for vaccines against pneumonia, diarrhoea and cervical cancer, GSK will support developing country governments as they transition to financing the full cost of their local vaccination programmes.
back to top 

The crossover round is alive and well. Just a few days after Dermira put out the word that a consortium of backers had come up with a $51 million C round, the company rolled out a $75 million IPO. And the news, along with a fresh, $86 million filing from Rhythm Pharmaceuticals, helps set the stage for a new round of fall biotech IPOs that will once again test investors’ appetite for risk.
First, let’s look at Redwood City, CA-based Dermira. Focused on skin ailments, the biotech is partnered with UCB on the development of Cimzia–already on the market–for psoriasis, a field that will soon be packed with a host of contenders from the likes of Novartis ($NVS) and Eli Lilly ($LLY). The biotech has laid plans for a Phase III psoriasis trial in 2015 as it pursues further work on a new therapy for “hyperhidrosis,” or excessive sweating, in the armpits. It’s in a Phase IIb study and a successful outcome would set the stage for a late-stage program. There’s also an acne treatment in early mid-stage studies.
back to top 

The Maryland Department of Business and Economic Development says its Maryland Venture Fund has made follow-on investments in two Maryland startups totaling $700,000.
Bethesda-based KoolSpan, a mobile security encryption company, received $400,000. BrainScope, also in Bethesda, got $300,000. BrainScope is developing neurotechnology for quickly assessing traumatic brain injury.
back to top 

Emergent BioSolutions is looking to fill a position for a senior scientist position.
If hired, the senior scientist will be responsible for vaccine potency and assay development at Emergent BioSolutions.
Key responsibilities of the position include the design and execution of experimental protocols for developing and qualifying animal-based potency assays, conducting in vitro potency assay development and leading vaccine product stability studies with the company’s Potency Assay Group.
back to top 

Steve Blank, a serial entrepreneur, author and professor at Stanford University, developed the curriculum for the National Science foundation’s business boot camp for scientists before the NIH adopted it for scientists in the SBIR grant program. At a fireside chat at New York University Langone Medical Center hosted by NYC Bio this week, Blank talked about a new investment group called M34 Capital that will invest in technologies seeded by federally funded research, particularly graduates of I-Corps boot camps.
Led by CEO Errol Arkilic, founding and lead program director for the NSF I-Corps program, the Berkeley-based company was co-founded by Arkilic, Blank, Jim Hornthal, and Tom Baruch, according to its website. Its seed investment spans from $50,000 to $250,000. “We want to be the first external capital invested, and work closely with the founding team to help refine their business model and create the framework to systematically demonstrate its full potential,” according to the website. “We believe that the best entrepreneurs have the ability to blend technical insight with market-based feedback, allowing their innovations to mature into a successful company.”
back to top 

Howard University Hospital will receive $11 million in federal grants toward developing new therapeutics for the Ebola virus, Howard officials said.
At least $2 million from the National Institutes of Health will go toward developing a new drug to target a particular protein of the Ebola virus. As well, $2 million will go toward work at the hospital researching HIV resistance in people with sickle cell disease, officials said.
back to top 

BHC Center of Financial & Investment Business (“BHC”), an Atlanta based affiliate of Bao Hao Investment Management Co., Ltd. of Shanghai, has announced the first of what it expects to be several equity investments in 20/20 GeneSystems, Inc. (“20/20”). In making the investment BHC is seeking to help advance the introduction in China of 20/20’s blood test for the early detection of lung cancer (see www.BloodTestforLungCancer.com).
20/20 currently produces and markets this innovative blood test for the early detection of lung cancer in the United States. Primary care physicians employ the blood test to help detect lung cancer in patients who are smokers and former smokers. It is known as “PAULAs test.”
back to top 

With a massive influx of new funding, Frederick pharmaceutical company Vaccinogen Inc. is preparing to make big moves that leaders hope will put it on the map.
Vaccinogen announced Aug. 25 it had secured $80 million in funding from Stockholm investors and closed the first $10 million tranche of the funding. The funding will support late-stage clinical studies needed to make Vaccinogen’s OncoVAX commercially available. The drug is designed to reduce recurrence of colon cancer.
back to top 

ATCC, the premier global biological materials resource and standards organization, and bioMérieux, a world leader in the field of in vitro diagnostics, announced today their collaboration to expand and solidify the bioMérieux VITEK® MS microbial identification database for the industrial market. Recently, ATCC acquired the bioMérieux VITEK® MS system to make this innovative MALDI TOF technology a fundamental component of their authentication and quality control processes.
Strengthening the database with additional strains continues to increase the robustness of the VITEK® MS micro-organisms library while increasing the size and enhancing the utility for customers as a solid quality assurance tool.
back to top 

Venture capital (VC) funding for the Life Sciences sector, which includes Biotechnology and Medical Devices, reached $2.5 billion in 195 deals for the second quarter of 2014, according to a new report, “Biotech soars to record high,” which includes data from the MoneyTree™ Report from PricewaterhouseCoopers (PwC) LLP and the National Venture Capital Association (NVCA), based on data provided by Thomson Reuters. While this quarter was the highest Life Sciences investment since the second quarter 2007, it was also the strongest second quarter since 1995, which is the earliest data recorded by the MoneyTree.
back to top 

The National Institutes of Health is challenging science innovators to compete for prizes totaling up to $500,000, by developing new ways to track the health status of a single cell in complex tissue over time. The NIH Follow that Cell Challenge seeks tools that would, for example, monitor a cell in the process of becoming cancerous, detect changes due to a disease-causing virus, or track how a cell responds to treatment.
back to top 

A lot has been written lately about innovation, or the lack thereof, in the world of biopharma. One question that often gets asked: which countries lead the way in creating new medicines? Many people think that drugs originate in the nation where the companies that produce them are headquartered. The truth, however, is much more complicated. Given that multi-national firms market the majority of medicines, figuring out where each one of their drugs originated requires digging through some extensive data vaults. A proper analysis requires the examination of company histories, free market deal making, and in some cases government interventions. Consider the following examples:
back to top 

Student consultants at the University of Maryland’s Robert H. Smith School of Business often find their target audience staring at them in the mirror when they do market research for nonprofit organizations.
ChangeTheWorld.org, a pro bono consulting program launched at the school’s Center for Social Value Creation in 2006, reports an increased interest among nonprofit organizations in the Millennial Generation as the population segment grows up and goes to college. “Nonprofit organizations want to unleash the potential of this generation, and they are coming straight to the source for insights at the Smith School and other partner universities,” said Pammi Bhullar, program manager at the Center for Social Value Creation and ChangeTheWorld.org. “Essentially, they are asking college students to gather market intelligence on themselves.”
back to top 

A candidate Ebola vaccine could be given to healthy volunteers in the United Kingdom, The Gambia, and Mali as early as September, as part of a series of safety trials of potential vaccines aimed at preventing the disease that has killed more than 1,400 people in the current outbreak in West Africa.
Human trials of this candidate vaccine, being co-developed by the National Institutes of Health (NIH) and GlaxoSmithKline (GSK), are to be accelerated with funding from an international consortium in response to the Ebola epidemic, which the World Health Organization (WHO) recently declared a public health emergency of international concern.
back to top 

GlaxoSmithKline’s experimental Ebola vaccine could be tested on humans in the UK, US, the Gambia and Mali in the next few weeks, in a race to contain the deadly virus that has claimed more than 1,500 lives in west Africa.
The news came as the World Health Organisation (WHO) warned that the Ebola epidemic could eventually exceed 20,000 cases. Bruce Aylward, WHO’s assistant director-general for emergency operations, said: “This far outstrips any historic Ebola outbreak in numbers. The largest outbreak in the past was about 400 cases.”
back to top 

Mayor Stephanie Rawlings-Blake on Thursday appointed six new members to the Baltimore Development Corp. board, an announcement that comes as Councilman Bill Cole takes over as president of the agency.
The six new members are mostly long-standing vacancies on the 19-member board. One of the appointments fills a seat opened after city Finance Director Harry Black announced last month that he was leaving his job to become city manager in Cincinnati.
back to top 

Scientists who use government money to conduct genomic research will now be required to quickly share the data they gather under a policy announced on Wednesday by the National Institutes of Health.
The data-sharing policy, which will take effect with grants awarded in January, will give agency-financed researchers six months to load any genomic data they collect—from human or nonhuman subjects—into a government-established database or a recognized alternative.
back to top 

Have you always wondered what type of scientific researcher you are? Take the quiz to find out.
back to top 

Baltimore is going to be OK, as long as it has its big name medical centers.
That’s the big takeaway from a new report by Moody’s Investors Service that looked at the economic impact large medical and education systems have on the cities where they are based.
back to top 
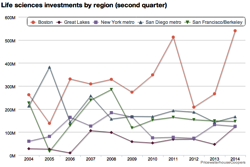
Venture capital continued its love affair with biotechnology in the second quarter, with investment in biotech reaching record levels.
Venture capitalists invested $1.84 billion in 122 biotech deals in the period, according to a MoneyTree report released Monday by PricewaterhouseCoopers and the National Venture Capital Association, using Thomson Reuters data.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Notice to Extend the Expiration Date of PAR-11-315 “Systems Science and Health in the Behavioral and Social Sciences (R21)”
- Notice to Extend the Expiration Date of PAR-11-314 “Systems Science and Health in the Behavioral and Social Sciences (R01)”
- Notice to Extend the Response Date for NOT-HL-14-028 “Request for Information (RFI): Collaborative Translational Research Consortium to Develop T4 Translation of Evidence-based Interventions”
- Notice of NLM’s Participation in PAR-13-358 “Opportunities for Collaborative Research at the NIH Clinical Center (U01)”
- Prize Competition: Challenges in Single Cell Analysis
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

On September 14, 2007, Lorenz Diesbergen, age 44, stepped off a commuter train in downtown Chicago and began his daily walk to work in the Chicago Loop. As he crossed the bridge over the Chicago River, his heart’s normal rhythm suddenly deteriorated into an uncoordinated frenzy of useless fibrillations. He may have managed a few more steps—we don’t know—before he pitched forward and fell face-first onto the sidewalk. Paramedics were on the scene within minutes, but efforts at resuscitation proved futile. He left behind a wife and four children.
back to top 

Bayer HealthCare appears to be the first pharmaceutical company that started its own accelerator. The company that made €18.924 million in turnover in 2013 “understands that innovation and healthcare cannot only be driven by the industry but also needs creativity.” This was announced by Christian Ullrich, Head of Marketing & Sales IT at Bayer HealthCare yesterday.
Bayer has partnered with universities and smaller companies before and started Grants4Apps, a crowdsourcing initiative last year. This year, it was turned into an accelerator.
back to top 

Tue, Sep 9, 2:00 PM – 3:00 PM
Hosted by NHLBI
In this Hangout, we will discuss the basic concepts of medical device regulation in the U.S., what is considered significant risk versus non-significant risk, and how the level of risk factors into the regulation of new product development.
back to top 

Get on the Bus!
Are you an entrepreneur ready to pitch your company? Register for your chance to film a 3-minute video pitch that will be seen online by VC, angel investors and voted on by fans around Maryland!
The Montgomery County Department of Economic Development and the Montgomery County Chamber of Commerce are hosting two Pitch Across Maryland stops in Montgomery County on Wednesday, October 1. Learn More Want to get on the bus and film your pitch? Apply Here.
The Montgomery County application deadline is Monday, September 15.
back to top 

Tuesday, December 2, 2014 from 7:00 AM to 7:00 PM (EST)
The 7th Annual Maryland Stem Cell Research Symposium, hosted by the Maryland Stem Cell Research Commission and Montgomery County will be held on Tuesday, December 2, 2014 at the Silver Spring Civic Center, Silver Spring MD. The Symposium is the Maryland Stem Cell Research Fund premier event that delivers comprehensive scientific talks, poster presentations, and networking time, enabling cell therapy basic research and technologies from the lab to pre-clinical and to commercialization. With a powerful line-up of speakers and many opportunities for you to present your work in concurrent or poster presentations, the Symposium will follow the format and style of previous meetings with additional networking time and an intimate environment.
Abstract Submission:
The Maryland Stem Cell Research Fund (MSCRF) is now accepting abstract submissions for poster and oral presentations for the 7th Annual Maryland Stem Cell Research Symposium. We encourage you to take full advantage of this opportunity to share your research results with our community. Multiple abstract submissions are welcomed.
ABSTRACT SUBMISSION DEADLINE: Wednesday, September 10, 2014
Please Click Here to submit your abstract today!
back to top 

The National Science Foundation (NSF) announced today that it will provide a multi-million dollar grant to create a hub of innovation that unites public and private institutions throughout Southern California, headquartered at and administered by the University of Southern California, the California Institute of Technology (Caltech), and the University of California Los Angeles.
The new center is part of the NSF Innovation Corps, or “I-Corps,” initiative, which is aimed at fostering innovation throughout the U.S. by encouraging the translation of ideas and research beyond the laboratory to create social and economic impact. The announcement cements the position of Southern California as a crucial focal point of technology entrepreneurship in the country.
back to top 

Too much fat weighs down not just your body, but also your brain.
harms most organs in the body, and new research suggests the brain is no exception. What’s more, the researchers found that getting rid of excess fat actually improves brain function, reversing the ill effects of the extra weight. The new study, which focused on people who underwent bariatric surgery, found that the procedure had positive effects on the brain, but other research has shown that less invasive weight loss strategies, like exercise, can also reverse brain damage thought to be related to body fat.
back to top 

Johnson & Johnson and Sanofi are using IBM Watson’s computer brain/big data cruncher to support research and development. It will be used to identify new applications for drugs that have already been developed and to leaf through scientific papers that detail clinical trial outcomes, according to a statement from IBM. The partnerships follow a new development in Watson’s evolution that help it visually uncover patterns and pinpoint connections in related data to accelerate the discovery process and advance science research.
“Watson now has the ability to understand the language of chemistry, biology, legal and intellectual property, giving scientists the ability to make connections with data that others don’t see, which can lead to rapid breakthrough in discoveries,” the statement said.
back to top 

Every day our DNA breaks a little. Special enzymes keep our genome intact while we’re alive, but after death, once the oxygen runs out, there is no more repair. Chemical damage accumulates, and decomposition brings its own kind of collapse: membranes dissolve, enzymes leak, and bacteria multiply. How long until DNA disappears altogether? Since the delicate molecule was discovered, most scientists had assumed that the DNA of the dead was rapidly and irretrievably lost. When Svante Pääbo, now the director of the Max Planck Institute for Evolutionary Anthropology in Germany, first considered the question more than three decades ago, he dared to wonder if it might last beyond a few days or weeks. But Pääbo and other scientists have now shown that if only a few of the trillions of cells in a body escape destruction, a genome may survive for tens of thousands of years.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

The 2014 Steering Committee of the Montgomery County Executive Hispanic Gala (MCEHG) announces the first of three awards to be presented during the gala celebration on September 18, 2014 to take place at The Fillmore Silver Spring.
University of Maryland President, Dr. Wallace D. Loh, will be the 2014 recipient of the “Advocate of the Year Award”.
back to top 

Location: MD, Gaithersburg – Corporate Headquarters
This will be a highly visible position dedicated to ensuring MedImmune further builds its collaboration efforts with governments, universities and non-profit organizations. The primary objective of this role will be to further entrench MedImmune in key biotech clusters to gain access to leading science and technology, enhance MedImmune’s reputation and create operational flexibility. The role will entail scouting for cross-therapeutic scientific collaboration opportunities, negotiating deals, working closely with internal scientists, event planning and strategic planning and analysis.
back to top 

MorphoSys AG (MOR) MPSYY, and Emergent BioSolutions Inc. EBS, today announced an agreement for the joint development and commercialization of ES414. The compound, to be renamed MOR209/ES414, is an anti-PSMA/anti-CD3 bi-specific antibody targeting prostate cancer, which was developed by Emergent using its proprietary ADAPTIRTM (modular protein technology) platform. Preclinical in vitro and in vivo studies have shown that MOR209/ES414 redirects T-cell cytotoxicity towards prostate cancer cells expressing Prostate Specific Membrane Antigen (PSMA), an antigen commonly found on such cells.
back to top 
The 2014 International Biotech Summit Committee invite BioHealth Innovation (BHI) and Montgomery County to attend the three co-located international conferences that will be held on Sep 9 -12 in Shenzhen, China:
- 2014 International Biotech Summit (September 10, 2014);
- The 9th International Conference on Genomics (ICG-9) (September 11th-12th, 2014);
- Shenzhen International Biotech & Health Industry Expo 2014 (SIBHIE, September 10th-12th, 2014).
Ms. Lily Qi, Director of Special Projects for Montgomery County Executive Office who was involved in establishing BHI and a native of Shanghai, is going to speak on behalf of the County and BHI for the forum, “Innovation in Policy and Regulation for Advancing Healthcare Industry.” BHI and the County are also invited as an exhibitor for the SIBHIE Expo.
back to top 

BioHealth Innovation, Inc. (BHI), a Montgomery County innovation intermediary which translates market-relevant research into commercial success by bringing together among other things management, funding, and markets, is seeking an experienced life science professional with entrepreneurial experience to serve as an Entrepreneur-in-Residence (EIR).
The EIR will lead in the evaluation of early-stage technologies and corporations with a priority focus on those associated with Funding Partners and those that best fit with the strategic direction as set by BHI’s CEO and Director of EIR Programs. The EIR will perform any reasonable task which will forward the goals of BHI. Further specifics and responsibilities include:
back to top 

A team of Biomedical Engineering undergraduate students from Johns Hopkins recently won the 2014 Design by Biomedical Undergraduate Teams (DEBUT) Challenge for developing a device that aims to help surgeons safely and accurately place screws during spinal fusion procedures and surgeries for spinal abnormalities.
The AccuSpine pedicle probe was designed by eight undergraduates: Clay Andrews, Eric Xie, Adarsha Malla, Bradley Isaacs, Anvesh Annadanam, Erica Schwarz, Ravi Gaddipati, and Luis Herrera. Their project began in July 2013 as part of a Center for Bioengineering Innovation and Design design team class. Their goal was to address a clinical need for an effective guidance system for the safe and accurate placement of screws.
back to top 

DATE: September 10, 2014 – 3:30 – 5:00 PM
LOCATION: Germantown Innovation Center, 20271 Goldenrod Lane, 2nd Floor, Germantown, MD 20874
As a federal agency, the Department of Energy is creative and innovative. Unlike other federal laboratories, each contractor-operated laboratory manages its own tech transfer agreements. Learn how to navigate these various opportunities and identify the best way to gain access to the technologies and partnerships to enhance your companies growth.
David E. Koegel
David Koegel is the senior technoogy transfer advisor in the Office of Science (SC) at the Department of Energy. A member of the Department’s Technology Transfer Policy Board, his experience in technology transfer goes back to the early 90’s when he was the Office’s Program Manager for American Textile partnership in the Laboratory Technology Transfer Program, a technology transfer collaboration among members of the integrated textile industry, the DOE national laboratories, a number of universities, and several research/education/technology transfer organizations. A member of the National Advisory Council of the Federal Laboratory Consortium, David has been the Department’s representative to the FLC for many years, as well as their representative to the federal Interagency Working Group on Technology Transfer. One of his significant duties involves the annual appraisal of the ten Office of Science national laboratories and is the primary point of contact for the various programs and organizations that use the laboratories’ personnel and facilities. Previously, he had been the Department’s Senior Technology Analyst in the SBIR/STTR Program, the Office of Science Small Business Program Manager, as well as the Program Manager for the Advanced Energy Projects program.
David joined the Department after spending several years at the Pentagon in the Office of the Assistant Secretary of the Army for Research, Development, and Acquisition, most notably during Desert Shield/Desert Storm, acting as a liaison to the Army’s laboratory system. Prior to this, he conducted research at the Army’s Night Vision Laboratory using metal organic chemical vapor deposition for the development of advanced infrared imaging devices.
David earned a B.S. in Chemical Engineering and an M.S. in Materials Science Engineering.
back to top 

The Maryland Technology Development Corp. is launching a new investment fund dedicated to cyber security.
The fund will make investments of up to $100,000 in companies that are developing and commercializing new cyber security projects.
back to top 

Roche Holding AG said Sunday it would pay $8.3 billion for a California biotech firm that has yet to turn a profit on a new drug to treat a deadly lung disease—the latest gamble by a pharmaceutical giant to buy its way into a lucrative corner of the industry.
The takeover would allow the Swiss company to expand its presence in the treatment of respiratory disorders, one of the world’s biggest drug markets. Roche’s offer of $74 a share represents a 38% premium over InterMune’s closing share price on Friday of $53.80, and a 63% premium before takeover speculation surrounding the biotech started circulating this month.
back to top 

Roche has just announced the acquisition of the U.S. biotech company InterMune for $8.3 billion. The driver for Roche was to gain access to InterMune’s drug, pirfenidone (trade name, Esbriet), which is already approved in Canada and Europe for idiopathic pulmonary fibrosis (IPF). IPF is a deadly lung-scarring disease which affects 50,000 – 70,000 people in the U.S. and 80,000 to 110,000 in Europe. While still under review by U.S. regulatory authorities, Roche’s acquisition signals its belief that pirfenidone will also gain U.S. approval. How big a product can pirfenidone be? Consensus forecasts compiled by Thomson Reuters Pharma peg annual sales at greater than $1 billion in 2019 – a nice addition to Roche’s stable of pulmonary compounds including Xolair (asthma) and Pulmozyme (cystic fibrosis).
back to top 

The Daily Record announced Thursday its 2014 Innovators of the Year, celebrating Marylanders and Maryland-based companies for their innovative spirit.
Twenty-eight innovators will be celebrated during the awards event on Oct. 15 at the American Visionary Art Museum, at 800 Key Highway in Baltimore.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
Updated Forms and Instruction Clarification for Re-entry (PA-12-150) and Diversity (PA-12-149) Administrative Supplements
(NOT-OD-14-118) National Institutes of Health
Small Business Innovation Research (SBIR) Program Contract Solicitation (PHS 2015-1) Now Available
(NOT-OD-14-120) Office of the Director, NIH
Notice of Participation of NLM in PAR-13-357 “Pre-application: Opportunities for Collaborative Research at the NIH Clinical Center (X02)”
(NOT-LM-14-003)
National Library of Medicine
Requests for Applications:
Multi-Site Clinical Trials for the Pulmonary Trials Cooperative (PTC) (U01)
(RFA-HL-15-015)
National Heart, Lung, and Blood Institute
Application Receipt Date(s): October 20, 2014
Network Management Core (NEMO) for the Pulmonary Trials Cooperative (PTC) (U01)
(RFA-HL-15-016)
National Heart, Lung, and Blood Institute
Application Receipt Date(s): October 20, 2014\
Program Announcements:
Administrative Supplements for Tobacco Regulatory Research on the Role and Impact of Flavors in Cigarettes, Cigars, E-Cigarettes and Smokeless Tobacco (Admin Supp)
(PA-14-320)
Office of the Director, NIH
National Cancer Institute
National Heart, Lung, and Blood Institute
National Institute on Alcohol Abuse and Alcoholism
Eunice Kennedy Shriver National Institute of Child Health and Human Development
National Institute on Drug Abuse
Office of Disease Prevention
Application Receipt/Submission Date(s): Multiple dates, see announcement.
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

JMI Equity closed a $1 billion growth equity fund targeted at investing in software companies and services companies, it announced Wednesday.
The fund is the largest raised by JMI, a private equity firm operating out of offices in Baltimore and San Diego. The firm has raised more than $3.1 billion in committed capital since its founding in 1992. The largest fund JMI previously closed on was an $875 million fund completed in November 2010.
back to top 

When you think of tech companies, incubators or startups, South Carolina is still probably one of the last places that comes to mind. But with its new Office of Economic Engagement, which launched in the summer of last year, and a steadily growing incubator program, the University of South Carolina is working to change any misconceptions about tech startups not meshing well with the Palmetto State.
Just last week, USC’s Technology incubator held a graduation ceremony for seven companies that were ready to transition into the larger business community.
back to top 

An invasive species has been introduced into the U.S. health innovation ecosystem, with a growing danger of permanent damage to the development of specialty drugs. The relentless assault on the price of Sovaldi is becoming a threat to the 30-year political balance that has energized the biomedical revolution.
Sovaldi is the kind of medicine that the drug scolds claim to want—a true scientific advance with a near-perfect cure rate for Hepatitis C, the liver-destroying virus that infects one of every 100 Americans and some 150 million world-wide. The old critique was that pharmaceutical discovery had stalled and the industry produced only me-too drugs. Now they attack Sovaldi because its price is $84,000 a patient.
back to top 

There are approximately 1,250 business incubators in the United States, including about 120 with some life science focus, according to the National Business Incubation Association (NBIA). These programs vary greatly in their structure and services.
Often associated with universities or medical centers, life science incubators frequently offer affordable office space attached to shared wet lab facilities. Others focus on accelerating companies’ progress by offering intensive coaching, networking and support services. Some programs include seed funding up to $250,000 at a stage when few others are willing to invest.
back to top 

Digital health incubator Rock Health is investing in eight new startups and teaming up with three new corporate entities – Blue Shield of California, Deloitte and global healthcare firm Abbott.
The new startups being incubated span the healthcare spectrum, from HIPAA compliance to life sciences and biotech. Among them:
back to top 

The National Science Foundation (NSF) has awarded 36 Early Concept Grants for Exploratory Research (EAGER) to enable new technologies to better understand how complex behaviors emerge from the activity of brain circuits.
These awards will contribute to NSF’s growing portfolio of investments in support of President Obama’s BRAIN Initiative, a multi-agency research effort that seeks to accelerate the development of new neurotechnologies that promise to help researchers answer fundamental questions about how the brain works.
back to top 
In July, alongside the ÜberResearch team, we launched a Twitter competition open to PhD students. The task was to tweet how you would spend £10 million of science funding using the hashtag #uberresearchprize.
We had hundreds of tweets and the top three, as voted by our judging panel, were then invited to write a blog post, delving into their original tweet. Over the next few days we will publish the top three blog posts here on our blog, announcing the winner on Monday 25th August.
back to top 

The interest in using health IT tools as a way to improve healthcare delivery and efficiency has produced many rapidly growing healthcare companies, many of which can trace their origins within one or two years of Obamacare’s passage. These companies have reached a stage of their development to accelerate growth and that has coincided with a readiness by healthcare providers and payers to adopt or ramp up their technology.
With a nod to the top healthcare company on Inc’s 5,000 Fastest Growing Companies, molecular diagnostic companies with smartphone-enabled technology have also become more attractive as investment and acquisition targets.
back to top 

Tuesday, December 2, 2014 from 7:00 AM to 7:00 PM (EST)
The 7th Annual Maryland Stem Cell Research Symposium, hosted by the Maryland Stem Cell Research Commission and Montgomery County will be held on Tuesday, December 2, 2014 at the Silver Spring Civic Center, Silver Spring MD. The Symposium is the Maryland Stem Cell Research Fund premier event that delivers comprehensive scientific talks, poster presentations, and networking time, enabling cell therapy basic research and technologies from the lab to pre-clinical and to commercialization. With a powerful line-up of speakers and many opportunities for you to present your work in concurrent or poster presentations, the Symposium will follow the format and style of previous meetings with additional networking time and an intimate environment.
Abstract Submission:
The Maryland Stem Cell Research Fund (MSCRF) is now accepting abstract submissions for poster and oral presentations for the 7th Annual Maryland Stem Cell Research Symposium. We encourage you to take full advantage of this opportunity to share your research results with our community. Multiple abstract submissions are welcomed.
ABSTRACT SUBMISSION DEADLINE: Wednesday, September 10, 2014
Please Click Here to submit your abstract today!
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 27
American Tap Room

September 10-12
Shenzhen Convention & Exhibition Center

September 10
Shenzhen Convention & Exhibition Center

September 10-12
Washington, DC | San Francisco, CA

September 10
Germantown Innovation Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI) announced the appointment of four new members to its Board of Directors. Dave Lemus, Chief Executive Officer, Sigma-Tau Pharmaceuticals Inc.; Joel S. Marcus, Chairman, Chief Executive Officer, and Founder, Alexandria Real Estate Equities, Inc. (Alexandria Venture Investments); and Beth Meagher, Principal, Deloitte Consulting LLP, have joined the BHI Board of Directors, effective August 7, 2014, based upon their organizations’ newly committed support of BHI. Charles “Chuck” Morton now represents Venable LLP on the Board, as another new Board member, while Mike Baader, formerly with Venable, will continue to hold a seat on the Board, but now as a representative of Greenspring Associates. Cynthia L. Collins, CEO, Clarient, who had served on the BHI Board since early 2013, is stepping down from her Board position after relocating to the West Coast
“The expansion of and continued diversity within the BHI Board, based upon new key industry and service provider representatives who are all deeply involved in life sciences, is a testament to the positive impact BHI has made on the Central Maryland biohealth community to date,” said Richard Bendis, BHI President & CEO.
“We look forward to the contributions from the new Board members representing these organizations. We’re also pleased to welcome a new Venable representative to the Board and that Mike Baader will continue to add value to the organization now that he is on the Greenspring Associates team,” said Douglas Liu, Chair, BHI Board of Directors, and Senior Vice President of Global Operations for Qiagen. “And, on behalf of the BHI staff and Board, I would also like to offer gratitude to Cynthia Collins for her service and contributions to the BHI Board over the last year and half.”
back to top 

BioHealth Innovation, Inc. (BHI), a Montgomery County innovation intermediary which translates market-relevant research into commercial success by bringing together among other things management, funding, and markets, is seeking an experienced life science professional with entrepreneurial experience to serve as an Entrepreneur-in-Residence (EIR).
The EIR will lead in the evaluation of early-stage technologies and corporations with a priority focus on those associated with Funding Partners and those that best fit with the strategic direction as set by BHI’s CEO and Director of EIR Programs. The EIR will perform any reasonable task which will forward the goals of BHI. Further specifics and responsibilities include:
back to top 
The 2014 International Biotech Summit Committee invite BioHealth Innovation (BHI) and Montgomery County to attend the three co-located international conferences that will be held on Sep 9 -12 in Shenzhen, China:
- 2014 International Biotech Summit (September 10, 2014);
- The 9th International Conference on Genomics (ICG-9) (September 11th-12th, 2014);
- Shenzhen International Biotech & Health Industry Expo 2014 (SIBHIE, September 10th-12th, 2014).
Ms. Lily Qi, Director of Special Projects for Montgomery County Executive Office who was involved in establishing BHI and a native of Shanghai, is going to speak on behalf of the County and BHI for the forum, “Innovation in Policy and Regulation for Advancing Healthcare Industry.” BHI and the County are also invited as an exhibitor for the SIBHIE Expo.
back to top 
Montgomery County Department of Economic Development (DED) announces the addition of Valerie Fremont as Senior Business Development Specialist for the County’s life sciences and health IT sectors. Fremont brings 13 years of industry experience to the County along with experience in the international, national and regional biotech community.
”We are happy to welcome Valerie to the department and fortunate to be able to call upon her vast experience in the life sciences arena,” said DED Director Steve Silverman. “Not only does she bring a wealth of knowledge of two of our most important sectors—life sciences and health IT—but she also is already well known in that community. The County and our companies in those sectors will all benefit mutually from those important connections.”
back to top 

August 20th, 3-5 PM
MdBio Foundation welcomes you to an open house on the mobile laboratory MdBioLab. Tour the lab, meet the team, or just eat your ice cream – doors open 3 – 5 PM.
KICK OFF THE SCHOOL YEAR WITH AN ICE CREAM CONE AND TOUR OF MDBIOLAB.
MdBioLab will be parked at Howard Community College, 10901 Little Patuxent Pkwy, Columbia, MD 21044, Lot F – adjacent to Little Patuxent Parkway
back to top 

Wrap up a great summer with one last BioBuzz networking event on August 27th from 5:00 – 7:30 p.m. at American Tap Room in Rockville, MD. This location is a short walk from the Metro located in the Rockville Town Center so there are no excuses not to come.
New this month, join us for Table Talks at BioBuzz.
This month’s featured Table Topics will be on Project Management & Navigating your Life Science Careers.
Life Science Career Talks with;
Shira Harrington, President of Purposeful Hire
Multiple corporate recruiters from local Biotech companies
Project Management discussions with;
Tariq Allana, Project Manager at GlycoMimetics
Allen Bolden, Experienced GMP Manufacturing Project Manager
back to top 

Sigma-Tau Pharmaceuticals (Sigma-Tau) has signed an exclusive US license agreement with Ireland-based Crosscare, to market and distribute Colief Infant Drops for the treatment of colic.
As part of the deal, Sigma-Tau will be responsible for the sales, marketing and distribution of Colief in the US.
Sigma-Tau has planned a re-launch of Colief at healthcare professionals as well as consumers in August 2014.
back to top 

The Rockville Science Center has found a temporary office home in the midst of an ongoing search for a permanent facility.
The science center’s administrative office, previously at the VisArts at Rockville center, moved this month to the Johns Hopkins University’s campus in Rockville.
back to top 

A unique summer internship program established by the Universities at Shady Grove (USG) and funded in part by the University of Maryland, Baltimore (UMB), has enabled 12 students to work across disciplines in a community setting.
Based at the Rockville, Md., campus where UMB offers a growing array of programs, the eight-week internship was conducted within the Montgomery County Department of Health and Human Services (DHHS). Four graduate and eight undergraduate students experienced interdisciplinary teamwork by visiting several service agencies throughout Montgomery County.
back to top 

Johns Hopkins University’s FastForward business accelerator is so popular the school is expanding it to a second location.
FastForward, which is part of Hopkins’ Whiting School of Engineering, launched last year and is already at capacity with a total of 33 startups and projects. Administrators plan to expand the program in January to accommodate the high demand among students and faculty.
back to top 

The BioMaryland Center is partnering with Maryland’s Department of Health and Mental Hygiene (DHMH) and the Center for Medical Technology Policy (CMTP) to incorporate improved health care quality and cost reduction criteria in the selection process for the BioMaryland Center’s annual Biotechnology Awards program. $1M will be awarded to projects, $50,000-200,000 each, advancing technologies toward commercialization–with preference given to projects which improve patient outcomes and reduce costs.
October 1st is the deadline to apply for the next round of Biotechnology Development Awards. More information regarding the program is available online.
How to Apply: Two application forms (the Non-confidential Applicant and Project Summary form and the Confidential Application Details form) and a business plan must be submitted electronically by 5 p.m. EST on October 1, 2014.
back to top 

The Maryland Venture Fund (MVF), the equity investment arm of the Maryland Department of Business and Economic Development (DBED), has made follow-on investments totaling $700,000 in two of its portfolio companies. KoolSpan, developer of a suite of patented, hardware-based mobile security encryption solutions, received $400,000. BrainScope, which is pioneering sophisticated neurotechnology to quickly assess traumatic brain injury at the initial point of care, received $300,000. Both companies are located in Bethesda. The investments were made with funds raised by InvestMaryland, Governor Martin O’Malley’s key initiative designed to spur growth and development of small, high-tech companies in Maryland.
“KoolSpan and BrainScope have both made tremendous progress since the Maryland Venture Fund first invested in them, and we are excited to make these follow-on investments,” said Governor O’Malley. “In Maryland we are committed to supporting the growth of future leaders in industries that keep us safer, healthier and better connected than ever before. KoolSpan and BrainScope are perfect examples of the innovative, high-tech companies that set Maryland’s economy apart and provide the family-sustaining jobs of today and tomorrow.”
back to top 

When four professors of the Indian Institute of Science (IISc), Bangalore, decided to start Strand Life Sciences in 2000 to aid clinical research through genomic testing using DNA sequencing, the initiative hardly attracted attention. UTI Ventures pumped in $1.3 million a year later and, in 2002, WestBridge Capital Partners invested $1.9 million, but that did not create ripples either. However, things changed dramatically in 2013, when Strand got $10 million from San Francisco-based financial firm Burrill & Co.
It used the capital to roll out personalised genetic test services in collaboration with hospital chains and clinics across India, including Max Hospitals, Mazumdar-Shaw Cancer Center, St. John’s Medical College Hospital, Kidwai Memorial Institute of Oncology and HealthCare Global Enterprises.
back to top 

GlaxoSmithKline, better known as GSK, has launched a new web effort aimed at providing both information and inspiration for people with chronic obstructive pulmonary disease, in what the company says is arguably the most engaging platform in the marketplace.
The rollout of the new COPD.com goes beyond what was previously offered, certainly by GSK but possibly many others, according to Darielle Ruderman, senior director of repository consumer marketing for the North Carolina-based company.
back to top 

The answers Bert Vogelstein needed and feared were in the blood sample.
Vogelstein is among the most highly cited scientists in the world. He was described, in the 1980s, as having broken into “the cockpit of cancer” after he and coworkers at Johns Hopkins University showed for the first time exactly how a series of DNA mutations, adding up silently over decades, turn cells cancerous. Damaged DNA, he helped prove, is the cause of cancer.
Now imagine you could see these mutations—see cancer itself—in a vial of blood. Nearly every type of cancer sheds DNA into the bloodstream, and Vogelstein’s laboratory at Johns Hopkins has developed a technique, called a “liquid biopsy,” that can find the telltale genetic material.
back to top 

DC I-Corps, a new, NSF-supported program designed to foster, grow and nurture an innovation ecosystem in the Mid-Atlantic Region, is now accepting applications for its fall cohort, beginning on October 9. Applications will be accepted on a rolling basis up until that date. Apply here.
Open to research teams and technology entrepreneurs from universities, federal laboratories, agencies and the general community, the free program guides researchers in exploring the commercial potential of their inventions.
Through DC I-Corps, you will:
- Significantly improve your chances for SBIR and other grant funding, as well as early stage venture investment;
- Work closely with six or more real-world advisors that have startup, venture capital, and technology commercialization experience over a six-week period; and
- Come to a clear go or no-go decision regarding the commercial potential of your technology.
The program is geared towards innovations coming from engineering fields, medical/health/life sciences, and physical and computer sciences. DC I-Corps builds upon the successful National Science Foundation (NSF) I-Corps program.
The program is jointly offered by the University of Maryland, George Washington University, Virginia Tech, and Johns Hopkins University. For more information and to apply, technology researchers and entrepreneurs are encouraged to visit www.dcicorps.org.
back to top 

Are you a company in the fields of cloud computing, network security, interoperability, chemical and biological defense, infectious disease or vaccines? Then you’ll want to attend the Maryland Department of Business and Economic Development’s Defense Labs Tech Transfer event which includes panel discussions and a tech transfer showcase. Hear from Federal Labs including NSA, DISA, RDECOM, Edgewood Chemical Biological Center (ECBC), and USAMRIID, see demonstrations, speak with inventors, discuss available patents, licenses, collaborations and commercialization of new technologies to add to your portfolio.
Featuring:
Tech Transfer Showcase 9:00 a.m. – 4:00 p.m. – Visit the exhibit floor and see the latest technologies being developed by NSA, DISA, RDECOM, USAMRIID, and the Edgewood Chemical Biological Center.
back to top 

Innovative technologies and methodologies fuel progress in biomedical and behavioral research and represent an increasingly important area of the economy. The Small Business Innovation Research (SBIR) program provides support for research and development (R&D) of new or improved technologies and methodologies that have the potential to succeed as commercial products.
The purpose of this notice is to (1) announce the issuance of the Solicitation of the National Institutes of Health and the Centers for Disease Control and Prevention for Small Business Innovation Research Contract Proposals (PHS 2015-1) with a receipt date of November 5, 2014, 4:30 PM ET; and (2) inform the public about the opportunities that the SBIR program offers to small business concerns as well as to scientists at research institutions.
The SBIR legislation requires the Public Health Service (PHS), Department of Health and Human Services, and certain other Federal agencies to reserve 2.8 percent (for FY 2014) of their extramural research or R&D budgets for an SBIR program. (The NIH SBIR set-aside requirement for FY 2014 is $663 million.)
back to top 

The National Institutes of Health is challenging science innovators to compete for prizes totaling up to $500,000, by developing new ways to track the health status of a single cell in complex tissue over time. The NIH Follow that Cell Challenge seeks tools that would, for example, monitor a cell in the process of becoming cancerous, detect changes due to a disease-causing virus, or track how a cell responds to treatment.
“Advances in cellular analysis promise earlier diagnosis and improved therapies for diseases, from cancer to Alzheimer’s,” said James Anderson, M.D., Ph.D., director of NIH’s Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI). “These prizes will also help to stimulate new businesses and economic growth in our biomedical communities.”
back to top 

For the third year in a row, Johns Hopkins has been recognized as one of the nation’s best college campuses for dining.
Hopkins ranks second on the list of the “Best Colleges for Food in America” compiled by the website The Daily Meal, up from a No. 42 ranking a year ago. The site praised JHU’s commitment to sustainability, the diversity of food offerings, and its special programming, including on-campus cooking classes and food and wine pairings for seniors.
back to top 

For an incubator set on changing perceptions of where startups need to be to succeed in regulated sectors such as healthcare, education and energy, the decision to set up 1776 in Washington D.C. was a smart move. Mentors abound in a city where lawmakers, think tanks, regulators and Fortune 500 companies come to do business. In a phone interview, co-founder Donna Harris talked to MedCity News about its approach to finding and working with startups.
Harris co-founded 1776 in 2012 with Evan Burfield. Harris had previously worked at Startup America Partnership (which later changed its name to UP America) as managing director, and before that she was Vice Chair of Interpoint. Burfield founded netDecide and Synteractive.
back to top 

Luminal, a Frederick and D.C.-based cloud management startup, on Thursday announced a $10 million Series B round led by New Enterprise Associates.
Joining NEA are prior investors Core Capital Partners and the Maryland Venture Fund. The latest financing brings Luminal’s total funding to $13.8 million.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- NIH Offers Commercialization Assistance Program to Phase II SBIR and STTR Awardees
- Revised Policy: Descriptions on the Use of Individual Development Plans (IDPs) for Graduate Students and Postdoctoral Researchers Required in Annual Progress Reports beginning October 1, 2014
- Notice of NICHD’s Participation in PA-14-016 “Ruth L. Kirschstein National Research Service Award (NRSA) Short-Term Institutional Research Training Grant (Parent T35)
- (NOT-HD-14-026)
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

The rich are getting richer when it comes to publicly traded biotechnology companies, judging by their ballooning market capitalization. It’s a product of the current Wall Street surge—some call it a bubble—that continued in the first half of 2013, with 52 companies going public and the overall market closing at record highs.
Following is a list of 25 biotechnology companies, ranked by their market cap as of July 24 as furnished by the exchanges on which they trade their shares, or by other publicly available sources, such as any of several free stock information websites. Figures of non-U.S. companies were converted to U.S. dollars from various currencies.
back to top 

Monday, September 22, 2014
NHLBI’s Office of Translational Alliances and Coordination (OTAC) hosts this semi-annual Regional Innovation Conference to connect small businesses, angel investors, venture capitalists, strategic partners, and business leaders from the biotech, medical device, and pharmaceutical industries. Attendees will see featured presentations from NHLBI-funded companies, and learn from staff about recent changes in the Federal SBIR/STTR program, and other funding opportunities and cost-free resources available to small businesses.
Previous conferences have been held in Boston, MA, San Francisco, CA, San Diego, CA, and Rockville, MD.
Highlights/ Features:
- Company presentations
- Hear about innovative technologies for heart, lung, blood and sleep disorder and disease indications from companies who have received (non-dilutive) SBIR/STTR funding
- How to market your innovation
- Our expert panel of investors, legal advisors, and successful entrepreneurs will talk about the highs and lows of early-stage life science investment
- Networking
- Ample opportunities to network with potential partners, investors, and NHLBI staff
back to top 

Today, the American Heart Association has launched the Chicago Open Innovation Challenge, a crowdfunding competition to uncover new innovative digital health tools to prevent or manage heart disease and stroke. The top three finalists will receive grants from the American Heart Association totaling $25,000 and a chance to present at the Heart Innovation Forum in Chicago on November 14, 2014. Each award winner will be featured on healthcare crowdfunding platform MedStartr.
Who Can Apply? Early-stage healthcare technology and life sciences companies with novel ideas that seek to help patients, providers or medical facilities meet the American Heart Association’s 2020 Impact Goal—to improve the cardiovascular health of all Americans by 20 percent while reducing death from cardiovascular diseases and stroke by 20 percent by the year 2020—are encouraged to apply.
back to top 

Traditional Chinese Medicine (TCM) has been used for thousands of years to prevent and treat disease and promote wellness. But, in many western countries, there had been historic resistance to its use because it “was not supported by rigorous scientific study.”
That’s not true today.
back to top 

Digital health has a pretty erratic grade point average, or so says Dana Mead, a parter at venture firm Kleiner Perkins, Caufield & Byers. He outlined and graded what he called the five most important elements of the digital health ecosystem at Qualcomm Life‘s Connect2014 conference this week in San Diego, and this is how he slices it:
1. Access to Capital – A
Looking at data over the last quarter, Mead said that about $700 million in funding has been raised in digital health. That’s more than medical device startups raise, about two-thirds of what pharma and biotech raise and about a third of what software/IT companies raised in the same time period. So, not a bad showing for digital health, Mead said.
back to top 

The organizers of the 2nd Annual Symposium for Pediatric Surgical Innovation and Competition judges are seeking proposals from inventors in medical institutions, private practices, device companies, and academic researchers who have medical device concepts or ideas for use with pediatric patients.
Proposals should address a significant, yet unmet need within the pediatric population with a device idea that lends itself to commercialization. Following the competition process, two prizes will be given, each for up to $50,000.
Please follow this link http://www.pediatric-surgery-symposium.org/prizes/ to review the submission guidelines and to submit your application online by September 22, 2014 at 17:00 EST.
back to top 

The mobile revolution was one of the most transformative events in IT. It set IT in a tailspin trying to secure so many different device types connecting to the network and moving outside of the organization.
After initial resistance, IT realized that by supporting mobility they were supporting user productivity. And thus an established industry of mobile device management solutions was born, allowing IT to create policies and strike a balance between data security and mobility.
back to top 

Much of the discussion on big data has focused on claims information from insurers and EHRS from providers, but a collaborative effort underway at Stanford Medical School with SAP is hoping to tap into a different set – genomic data.
The possible benefits of sharing genetic data on a wide scale have great potential for both global population health and for drug makers alike, said Dr. Carlos Bustamante, who heads the Department of Genetics at the Stanford School of Medicine. Benefits range from possibly learning why certain drugs never make it out of clinical trials because of what population they are tested on to a more inclusive global snapshot at differing populations and what drives their health spending, among others.
back to top 

The annual global confidence survey from the National Venture Capital Association produced an unusual apparent contradiction.
Investors around the world think the U.S. is the best place to back startups but confidence in our country’s government among domestic VCs is dropping.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 20
Howard Community College

August 27
American Tap Room

September 10-12
Shenzhen Convention & Exhibition Center

September 10
Shenzhen Convention & Exhibition Center

September 10-12
Washington, DC | San Francisco, CA
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
– Representatives from Sigma-Tau Pharmaceuticals Inc., Alexandria Real Estate Equities, Inc., and Deloitte Consulting LLP Take New Board Seats –
– Charles Morton Becomes New Venable LLP Representative on Board while Mike Baader Stays on as Board Member (Now as Greenspring Associates Representative) –

ROCKVILLE AND BALTIMORE, MARYLAND, August 11, 2014 – BioHealth Innovation, Inc. (BHI) announced today the appointment of four new members to its Board of Directors. Dave Lemus, Chief Executive Officer, Sigma-Tau Pharmaceuticals Inc.; Joel S. Marcus, Chairman, Chief Executive Officer, and Founder, Alexandria Real Estate Equities, Inc. (Alexandria Venture Investments); and Beth Meagher, Principal, Deloitte Consulting LLP, have joined the BHI Board of Directors, effective August 7, 2014, based upon their organizations’ newly committed support of BHI. Charles “Chuck” Morton now represents Venable LLP on the Board, as another new Board member, while Mike Baader, formerly with Venable, will continue to hold a seat on the Board, but now as a representative of Greenspring Associates. Cynthia L. Collins, CEO, Clarient, who had served on the BHI Board since early 2013, is stepping down from her Board position after relocating to the West Coast
“The expansion of and continued diversity within the BHI Board, based upon new key industry and service provider representatives who are all deeply involved in life sciences, is a testament to the positive impact BHI has made on the Central Maryland biohealth community to date,” said Richard Bendis, BHI President & CEO.
“We look forward to the contributions from the new Board members representing these organizations. We’re also pleased to welcome a new Venable representative to the Board and that Mike Baader will continue to add value to the organization now that he is on the Greenspring Associates team,” said Douglas Liu, Chair, BHI Board of Directors, and Senior Vice President of Global Operations for Qiagen. “And, on behalf of the BHI staff and Board, I would also like to offer gratitude to Cynthia Collins for her service and contributions to the BHI Board over the last year and half.”
back to top 

Montgomery County, MD DED Tech Transfer Programs Monthly Event – Second Wednesday of every month: 3:30 PM to 5:00 PM (EDT) Germantown, MD
back to top 

A few days after their state’s primary elections, Chinese-American community leaders in Maryland woke up to an email in their inboxes.
It was from Lily Qi – director of special projects for the government of Montgomery County, an affluent suburb of Washington – thanking them for their support in the reelection campaign of County Executive Ike Leggett, whose primary victory cleared the way for a third term.
“In a low turnout election like this one, every vote counts and the immigrant community holds great sway in tipping the balance,” Qi said in her note. Throughout the campaign, Qi had tirelessly reached out to the Chinese-American community, which accounts for 5 percent of
back to top 

Rockville-based electronic prescription software service provider DrFirst on Wednesday announced $10 million in debt financing from Silicon Valley Bank.
The health IT company has expanded its products over the years to include software in medication management, adherence and care coordination. The company said the financing will go toward product development and an anticipated international expansion in the next year.
back to top 

Emergent BioSolutions Inc. EBS -1.39% today announced that it has submitted a Biologics License Application to the U.S. Food and Drug Administration (FDA) for Anthrax Immune Globulin Intravenous (Human) [AIGIV] as part of a development contract with the Biomedical Advanced Research and Development Authority (BARDA). AIGIV, which was acquired in the Cangene acquisition completed earlier this year, is being developed as an intravenous therapeutic treatment for inhalation anthrax.
“This accomplishment is a testament to the diligent work of our employees within the recently acquired Cangene operations and their years of successful collaboration and partnering with the U.S. government,” said Adam Havey, executive vice president and president biodefense division at Emergent BioSolutions. “We commend BARDA for their steadfast commitment to advancing this key anthrax countermeasure program and remain dedicated to supporting their mission to protect our civilian and military population.”
back to top 

Getting a shot is only a few seconds of discomfort, but that’s the first line of defense against an infectious disease.
It’s a process Emergent Biosolutions in Lansing knows well, as the only company that produces a licensed anthrax vaccine.
“Phase one is safety, phase two is selection and dose ranging, phase three we try to evaluate does it really work do we get the immune response we want,” said President of the Biodefense Division at Emergent Adam Havey.
back to top 

Public health officials have just one tactic to battle the unrelenting Ebola virus outbreak in West Africa — quarantine — but as the disease continues to spread, scientists in Maryland are among those close to discovering other weapons.
Baltimore companies Profectus BioSciences and Paragon Bioservices, as well as researchers at the U.S. Army Medical Research Institute for Infectious Diseases at Fort Detrick in Frederick and the National Institutes of Health in Bethesda, have been part of efforts that have shown a handful of Ebola vaccine candidates are effective in monkeys.
back to top 

In the August 18, 2014 issue of Forbes Magazine, the University of Maryland, College Park was ranked as one of the nation’s most entrepreneurial research universities. These rankings are based off of the number of alumni and students who have identified themselves as founders and business owners on LinkedIn against the school’s total student body which includes undergraduates and graduate combined.
back to top 

Aspiring entrepreneurs in Maryland have two great options for higher education, according to ranking website College Choice.
The University of Maryland, College Park snagged the No. 20 spot on website’s list of the 50 best U.S. colleges for entrepreneurs, while Johns Hopkins University came in a bit lower, at No. 36.
back to top 

Johns Hopkins University is teaming up with Google on a project aimed at speeding up technology commercialization.
Under a new partnership agreement with Google, Hopkins will work on the search engine giant’s Advanced Technology and Projects (ATAP) group, which focuses on developing new technology and moving it quickly to the marketplace.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Notice on Annual Reporting Requirements and Revised Financial Closeout Requirements for NIH Administrative Supplements Awarded to Recover Losses Due to Hurricane Sandy under the Disaster Relief Appropriations Act
(NOT-OD-14-112) National Institutes of Health
- Notice of NHLBI Discontinuation of Grant Program “Ancillary Studies in Clinical Trials (R01)”
(NOT-HL-14-232)National Heart, Lung, and Blood Institute
- Notice to Extend the Response Date for the NOT-HL-14-030 “Request for Information (RFI): NHLBI Whole Genome Sequencing Project (NHLBI-WGS)”
(NOT-HL-14-233)National Heart, Lung, and Blood Institute
Program Announcements (PA):
- NHLBI Career Transition Award for Intramural Fellows (K22)
(PAR-14-302)
National Heart, Lung, and Blood Institute
Application Receipt/Submission Date(s): Multiple dates, see announcement.
back to top 

Wrap up a great summer with one last BioBuzz networking event on August 27th from 5:00 – 7:30 p.m. at American Tap Room in Rockville, MD. This location is a short walk from the Metro located in the Rockville Town Center so there are no excuses not to come.
New this month, join us for Table Talks at BioBuzz.
For a new twist to the networking events, starting this month we will be hosting “Table Talks at BioBuzz”. We have enlisted industry experts on various topics who will be joining us to facilitate discussions around their industry related topics of choice. They will be strategically situated at a few tables at our venue so that you will be able to join which ever conversations interest you throughout the evening and participate in some stimulating discussions; while still enjoying the usual, casual atmosphere that BioBuzz offers each month.
This month’s Table Topics and discussion leaders will be announced later this week so keep an eye out.
back to top 

At a laboratory in Baltimore, hairless mice kept in racks of plastic crates are labelled with yellow cards, each identifying a person fighting cancer. These mice are cancer “avatars”—the lumpy tumors visible under their skin come from actual patients.
The animals serve as personalized, living test tubes. Each mouse will eventually be treated with a different drug and its tumors measured. Results showing which medicine worked best will be sent back to a doctor trying to treat a difficult cancer case.
back to top 

The Swiss pharmaceutical giant Roche is set to pay $450 million for a Danish biotech company that develops drugs that silence microRNAs (something considered key to tackling certain diseases).
Roche is targeting the Danish biotech firm Santaris Pharma, according to The Wall Street Journal. Santaris Pharma A/S is a biopharmaceutical company founded in 2003 in Copenhagen. Santaris Pharma A/S has become a leading clinical-stage biopharmaceutical company that develops RNA-targeted medicines. Santaris works on developing drugs for a wide range of diseases using microRNA and mRNA. Their research focuses on infectious disease and metabolic disorders.
back to top 

The University of Maryland has launched an unmanned aircraft systems (UAS) test site in southern Maryland. With support from the University System of Maryland, the site will bring together leaders in academia, industry and government to accelerate UAS research.
Based in St. Mary’s County, a few miles from Naval Air Warfare Center Aviation Division at Patuxent River and the Naval Air Systems Command headquarters, the UMD UAS test site has been set up as a catalyst for research and development, according to its sponsors.
back to top 

ATCC, the premier global biological materials resource and standards organization, announced today that it has received ISO 13485:2003 certification for the development, manufacture, and distribution of standards, controls, reagents and in vitro diagnostic test kits.
Every day researchers in life science, medical diagnostics, applied sciences, and clinical drug development leverage ATCC’s expansive collection of cell lines, microorganisms, cellular and microbial panels, genomic and synthetic nucleic acids, biological products, and associated derivatives to generate quality research and reproducible results. ISO 13485 enhances ATCC’s existing Quality Management Program as an ISO 9001:2008 certified and ISO 17025:2005 and ISO Guide 34:2009 accredited organization.
back to top 

Wall Street may or may not be going through its long-awaited correction these days, but regardless, it still seems to have a hearty appetite for digital health initial public offerings. That appetite is provoking venture capitalists to fund ever-more startups. Through June of this year, $2.3 billion has been sunk into digital health offerings compared with $2 billion in all of 2013, reports digital healthcare accelerator Rock Health.
“We think there’s going to be four to five IPOs each year for the next five years.” said Steve Kraus, the lead healthcare partner at Bessemer Venture Partners, Cambridge, Mass.
back to top 
As a small company, you have a number of things going for you because of your size: You’re agile. You can react quickly to market changes. You can still innovate on a whim.
Big businesses know this all too well, which helps explain what’s driving an increase in the number of corporate venture capital arms cropping up in the past few years. If the old chestnut is correct, a rising tide floats all boats. So as the economy recovers and some sectors like technology seem like they’re heading toward irrational exuberance, corporate VC firms are eager to get in on the action.
back to top 

With an aging Baby Boomer population and broadband bandwidth improved a hundredfold from a decade ago, telemedicine is exploding as a convenient and less costly alternative to the traditional visit to the doctors’ office.
This year in the U.S. and Canada, 75 million of 600 million appointments with general practitioners will involve electronic visits, or eVisits, according to new research from Deloitte.
back to top 

Linda Wittbrodt was perusing Michigan Technological University’s website when she stumbled across a faculty project seeking funds to teach senior citizens online skills.
Wittbrodt thought that was cool, so she donated $25 through a new approach the Upper Peninsula university is using: pitching of an idea online and asking people to make a donation, a strategy known as crowdfunding.
back to top 

IT deal flow remained steady in 2Q. About $17 billion of PE capital was invested through 89 transactions, with both figures in line with previous quarters. The software segment of the industry is particularly active, with 2014 on pace to surpass the annual deal flow totals of any year following the financial crisis. Software has become industry-agnostic in some respects, as everything from colleges to hospitals to industrial conglomerates have incorporated software solutions into their operating models. For PE investors, software providers offer strong recurring revenue streams and also boast greater add-on potential for platforms.
back to top 

A study conducted by the Economic Alliance of Greater Baltimore Foundation (EAGB) to estimate Baltimore Gas and Electric’s (BGE) economic contributions to the region in 2013 found that its operations generated a total economic impact of $3.81 billion of output and supported 8,686 jobs. This resulted from the company’s direct effect, the effect of companies that provide services to BGE and the effect of employees of both BGE and the service companies. BGE’s activities contribute more than 2 percent of the entire economic output of the BGE service area in central Maryland.
“We are proud to be able to support our partners in their goal of measuring and understanding their importance to the region’s workforce and economy,” said Tom Sadowski, President and CEO of the EAGB. “Our Chief Market Analyst, Patrick Dougherty, did a tremendous job of assessing and articulating the impact of BGE’s economic contributions to the region.”
back to top 

A new collaboration between the USDA’s National Institute of Food and Agriculture (NIFA) Small Business Innovation Research Program (SBIR) and the USDA’s Agricultural Research Service (ARS) encourages SBIR applicants to license ARS technologies and be considered for a SBIR grant.
The relevant language in the SBIR’s “Request for Application” states: “Additional factors that will be considered in the review process include whether an application involves a CRADA with a USDA laboratory, or a license to a USDA technology, or is a resubmission. In the event that two or more applications are of approximately equal merit, the existence of a CRADA with a USDA laboratory or a license to a USDA technology will be an important consideration. If one application is a resubmission, this will also be an important consideration.” The SBIR Website can be found at: http://www.nifa.usda.gov/fo/sbir.
back to top 

Accelerator Corporation, a US-based biotech investment and management company, has attracted a total of $51.1m for its Accelerator IV fund. The three major investors’ cash, Eli Lilly, Pfizer Venture Investments and Johnson & Johnson Development Corporation, make up $50m of the investment.
The three pharmaceutical companies and other existing investors Alexandria Venture Investments, WRF Capital and Arch Venture Partners, are joined by two new strategic investors. These are Harris & Harris, a venture capital firm, and The Partnership Fund for New York City, an evergreen fund by the city’s business and finance leaders.
back to top 

Orgenesis Inc. (OTCQB: ORGS), a leader in the emerging fields of cellular therapy and re-generative medicine, today announced the appointment of industry veteran Scott Carmer as CEO of the company’s North American subsidiary. Carmer has more than 25 years of diverse industry experience within both pharmaceutical and biotech companies. Orgenesis is a pioneer in the field of “cellular trans-differentiation,” a technology that has potential to regenerate glucose-responsive insulin production and restore glycemic homeostasis for patients suffering from various insulin-dependent disorders. By transforming a patient’s own liver cells into new insulin producing cells, Orgenesis hopes to develop a breakthrough therapy for people living with Type 1 Diabetes. In his new role, Carmer will oversee the Orgenesis drug development and commercialization strategy in North America, focusing on the near-term initiation of Phase I and Phase II clinical trials in the United States.
back to top 

The National Center for Engineering Pathways to Innovation (Epicenter) is accepting proposals for its Pathways to Innovation Program.
The Pathways to Innovation Program is designed to help institutions fully incorporate innovation and entrepreneurship into undergraduate engineering education. The program is run by Epicenter, which is funded by the National Science Foundation and directed by Stanford University and the National Collegiate Inventors and Innovators Alliance (NCIIA).
back to top 

As the dust settles from this past week’s mammoth $1.3 billion merger, Siemens Health Services CEO John Glaser tells Healthcare IT News what led up to the Cerner deal, how his experience as a health system CIO could help smooth integration challenges and what to expect – from the two companies and electronic health records in general – over the months and years to come.
back to top 

Dr. Marvin Malek has been yearning and advocating for a publicly financed, single-payer health care system for at least two decades. Now, as Vermont stands on the threshold of being the first state to launch such a plan, he’s confessing to trepidation.
“I am pretty damn nervous,” he confided before bounding off for rounds at the Vermont Central Medical Center, still clutching the bicycle helmet he wore on his ride to work.
back to top 

Grand Challenges Canada, funded by the Government of Canada, has announced funding of seven projects implemented in ASEAN member countries. This funding, totalling $784,000, will support projects that combine scientific/technical, social and business innovation to solve pressing global health challenges.
The funding was announced by Canadian Foreign Affairs Minister John Baird during a visit to Burma, where he is participating in the ASEAN-Canada Post Ministerial Conference. As one of ASEAN’s longest-standing dialogue partners, Canada has enjoyed positive and fruitful relations with the ASEAN region, cooperating on many issues, including regional integration, economic interests and innovation.
back to top 
 Anne Wojcicki bounds into a conference room in Mountain View, California, straight from a five-mile ride from home on an elliptical bike. The 40-year-old cofounder and CEO of the consumer genetic testing firm 23andMe is breathless, and not just because of the workout. On this warm day in mid-June, Wojcicki is “super-excited” about an announcement scheduled for two days hence: the Food and Drug Administration has agreed to review a health-related genetic report the company wants to make available to customers. Anne Wojcicki bounds into a conference room in Mountain View, California, straight from a five-mile ride from home on an elliptical bike. The 40-year-old cofounder and CEO of the consumer genetic testing firm 23andMe is breathless, and not just because of the workout. On this warm day in mid-June, Wojcicki is “super-excited” about an announcement scheduled for two days hence: the Food and Drug Administration has agreed to review a health-related genetic report the company wants to make available to customers.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 13
Germantown Innovation Center

August 14
1776

August 14
Smokey Glen Farm Barbequers

August 27
American Tap Room

September 10-12
Shenzhen Convention & Exhibition Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

The Montgomery County Council gave final approval Tuesday to an ambitious land-use plan designed to spur creation of a new science-focused town center in the county’s long-neglected eastern sector.
The White Oak Science Gateway Master Plan envisions housing, retail and a hub for medical and life-sciences research adjacent to the Food and Drug Administration headquarters at Route 29 and Industrial Parkway. The plan also adjusts zoning and land-use regulations with the goal of energizing new residential construction and commercial renewal in the White Oak and Hillandale communities.
back to top 

What do you need to turn a brilliant idea into a business? “A good morale boost,” says Abhishek Motayed, Founder and President of N5 Sensors, Inc. of Rockville. Motayed had that boost this month when N5 Sensors received two Small Business Innovation Research (SBIR) awards totaling $250,000. The grants came from the Environmental Protection Agency (EPA) and the National Science Foundation (NSF) towards N5 Sensors work developing low-power, computer chip-size benzene, carbon monoxide, and ammonia sensors.
Image: http://umdtechtransfer.wordpress.com – Pasquale Ferrari, Ratan Debnath, Abhishek Motayed
back to top 

Johns Hopkins researchers Aleksander Popel and Jordan Green knew their research could serve a greater purpose outside their laboratory. But without any business experience, they knew they couldn’t do it alone.
With help from Hopkins’ tech transfer office and the Maryland Innovation Initiative, a state grant fund that invests in research projects with commercialization promise, the pair’s biomedical research is now a company — AsclepiX Therapeutics. The company is developing a better way to treat eye conditions caused by blood vessel abnormalities. Macular edema, which is common among people with diabetes and can lead to blindness, is an example.
Image: Jaclyn Borowski – At left, Niranjan Pandey, senior director of research and development, and Eric Bressler, research specialist, work in the lab at AsclepiX Therapeutics.
back to top 

Qiagen has agreed to partner with AstraZeneca to develop a liquid biopsy-based companion diagnostic test to accompany one of the pharmaceutical company’s lung cancer drugs.
Building on a master framework agreement signed by both companies in 2013, the partnership will involve the creation of a diagnostic test that analyses plasma samples to assess EGFR mutation status in non-small cell lung cancer patients.
back to top 

Opticul Diagnostics has won a BioMaryland Center grant to work on a medical device with a France-based company that would immediately identify microorganisms in a wound at a point-of-care setting, such as a hospital.
The device would be used on burn injuries and cutaneous wounds. The rapid identification of the bacteria in the wound would allow for quicker patient treatment.
Opitcul Diagnostics, based at Johns Hopkins University’s Montgomery County Campus, is collaborating with Diafir of Rennes, France. The two companies have received funding through a partnership between BioMaryland Center and Medicen Paris Region.
Image: http://mcc.jhu.edu
back to top 

Montgomery County, MD DED Tech Transfer Programs Monthly Event – Second Wednesday of every month: 3:30 PM to 5:00 PM (EDT) Germantown, MD
back to top 

Innovators and startups pride themselves on being creators of disruptive technologies. We expect them to introduce game-changing solutions that improve our quality of life and enable administrations to govern better.
But the journey from innovative solution to government adoption is far from easy and can seem impossible for smaller companies with cutting-edge technologies.
Image: http://www.statetechmagazine.com
back to top 

Regardless of your stance on Obamacare and Maryland’s Medicare waiver, you can’t ignore that health reform and technology are creating many opportunities for innovation. The BBJ is looking for those innovators. Please click below for contest information.
back to top 

SBA Awards Montgomery County Chamber Community Foundation grant
to conduct veteran small business training course
ROCKVILLE, MD – The Montgomery County Chamber Community Foundation (MCCCF) is pleased to announce that the U.S. Small Business Administration (SBA) will support MCCCF in providing technical training to veteran-owned businesses seeking federal procurement opportunities. Through a Cooperative Agreement with SBA, the Foundation’s National Center for Veteran Institute for Procurement (VIP) will expand and host three training sessions a year (over a twelve month period) to support up to 150 service-disabled veteran-owned small business (SDVOSB) and veteran-owned small businesses (VOSB) to attend the program.
back to top 

With plans to couple Johns Hopkins Medicine’s research cachet and Kaiser Permanente’s population health prowess, the two health giants announced a new “strategic collaboration” on Tuesday.
Kaiser Permanente of the Mid-Atlantic States and Hopkins officials say they will collaborate on patient care by sharing data from electronic medical records and developing better health care models based on evidence of what’s worked best. Kaiser will work closely with Suburban Hospital in Bethesda, which is part of the Johns Hopkins network.
Image: http://m.bizjournals.com
back to top 

A $10.7 million grant to the University of Maryland School of Dentistry and the University of Maryland School of Medicine will fund the collaborative study of biomarkers associated with sexually transmitted diseases such as chlamydia, in hopes of finding new ways to predict the infection and developing new vaccines or treatments. The five-year grant from the National Institute of Allergy and Infectious Diseases is the renewal of a previous $12 million, five-year grant awarded in 2009, bringing the project’s total to $22.7 million.
Image: http://www.oea.umaryland.edu
back to top 
In these times of tight budgets and rapidly evolving science, we must consider new ways to invest biomedical research dollars to achieve maximum impact—to turn scientific discoveries into better health as swiftly as possible. We do this by thinking strategically about the areas of research that we support, as well as the process by which we fund that research.
back to top 

Millions of people make a living without ever setting foot in an office. Particularly in technology, companies are moving away from just outsourcing rote tasks to remote workers and toward building entirely distributed teams. One leader is Elance-oDesk, the largest online marketplace for freelance talent. In addition to providing a platform for distributed and part-time work, the company practices what it preaches. Telecommuting in the United States increased by as much as 79% (paywall) between 2005 and 2012.
back to top 

The Global Innovation Index (GII) report, jointly produced by Cornell University, Insead and Wipo every year, has become a leading source of reference on innovation.
The theme of GII 2014, released on the 18th of July, is ‘Human factor in innovation’. The report covers 143 economies around the world and ranks them on a score of 0-100, using 81 different indicators to gauge innovation capabilities and results. Consistent with the rankings of the past GII reports, the top ten economies in the global innovation index 2014 are Switzerland, UK, Sweden, Finland, Netherland, USA, Singapore, Denmark, Luxemburg and Hong Kong (China), all high income countries, hence pointing towards a clear income-innovation link.
back to top 

According to the latest research out of Johns Hopkins, a new blood test could predict a person’s risk for suicide through their DNA. The blood test would rely on genetics and offer many who are afflicted with mental illness and their doctors a new option in detecting suicidal behavior.
back to top 

Genome scientist and entrepreneur J. Craig Venter is best known for being the first person to sequence his own genome, back in 2001.
This year, he started a new company, Human Longevity, which intends to sequence one million human genomes by 2020, and ultimately offer Web-based programs to help people store and understand their genetic data (see “Microbes and Metabolites Fuel an Ambitious Aging Project”).
Image: http://www.technologyreview.com – J. Craig Venter
back to top 

Education technology, or ed-tech, is getting big in Baltimore and local experts think Maryland has a shot at being a leader in this technology niche.
But it won’t happen overnight.
“I think we have a unique opportunity to build an ecosystem,” said Frank Bonsal III, an ed-tech venture capitalist who leads Towson University’s business incubator. “An ecosystem takes 20 years to build. We’re on year three.”
Image: Jaclyn Borowski – Andrew Coy, executive director of the Digital Harbor Foundation, says students should play a bigger role in growing ed-tech.
back to top 

When it comes to raising capital for a tech startup, savvy entrepreneurs know how important it is to hustle. From trying to attract the attention of VCs to appearing on television shows like ABC’s Shark Tank, these days when it comes to raising cash, everyone is out to get their share.
However, you may be surprised to learn that in this high-tech world, one very traditional institution is looking for smart entrepreneurs to give money to. Aspiring entrepreneur, meet the Small Business Administration (SBA).
back to top 

Maryland Health Secretary Dr. Joshua Sharfstein will step down in January to join Johns Hopkins University.
Sharfstein’s move comes as Maryland is trying to revamp its failed health exchange in time for November open enrollment and as Gov. Martin O’Malley’s administration winds down.
Image: Nicholas Griner Maryland Health Secretary Dr. Joshua Sharfstein is stepping down in January and will join Johns Hopkins University.
back to top 

The FLC planner visually communicates the outstanding research and development efforts of the federal laboratory system. Images and captions tell the story of the technology’s scientific relevance and potential impact.
Printed annually, the planner is distributed to over 10,000 recipients, including members of Congress, scientists, researchers, agency representatives, laboratory directors, technology transfer professionals, students, academia, and members of industry.
Image: http://www.federallabs.org
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Notice on Annual Reporting Requirements and Revised Financial Closeout Requirements for NIH Administrative Supplements Awarded to Recover Losses Due to Hurricane Sandy under the Disaster Relief Appropriations Act
(NOT-OD-14-112) National Institutes of Health
- Notice of NHLBI Discontinuation of Grant Program “Ancillary Studies in Clinical Trials (R01)”
(NOT-HL-14-232)National Heart, Lung, and Blood Institute
- Notice to Extend the Response Date for the NOT-HL-14-030 “Request for Information (RFI): NHLBI Whole Genome Sequencing Project (NHLBI-WGS)”
(NOT-HL-14-233)National Heart, Lung, and Blood Institute
Program Announcements (PA):
- NHLBI Career Transition Award for Intramural Fellows (K22)
(PAR-14-302)
National Heart, Lung, and Blood Institute
Application Receipt/Submission Date(s): Multiple dates, see announcement.
back to top 

The 2014 UEDA Awards of Excellence finalists have been chosen and the competition this year is at an all time high. Each year, universities and organizations across North America submit nominations for innovative programs that focus on developing economic prosperity in their communities and beyond. A panel university and economic development professionals have chosen 19 finalists from the group of nominated projects.
The UEDA awards are designed to help accelerate these programs by recognizing cutting edge initiatives, and to promote their adoption by other universities and communities. Program categories include: Community Connected Campuses, Innovation and Entrepreneurship, Leadership and Collaboration, Research and Analysis, Talent Development.
back to top 
The healthcare system in the U.S. is a slow-moving, slow-changing beast, but it’s also riddled with inefficient parts that beg for data-driven reinvention. That’s created huge opportunities for startups — and some of them are already seeing the payoff. The Affordable Care Act and accompanying legislation like the HITECH Act have lit a fire under the movement to rethink they way we deliver and pay for healthcare. Accordingly, investment in the digital health space has accelerated in 2014; at the end of June it totaled $2.2 billion, already exceeding 2013’s total funding just halfway through the year.
back to top 

Report: “Safe Science: Promoting a Culture of Safety in Academic Chemical Research”
Author: Committee on Establishing and Promoting a Culture of Safety in Academic Laboratory Research
Organization: National Research Council
Summary: The National Research Council formed a panel of university lab-safety experts after a series of campus accidents, including several deaths, emphasized that academic labs have a far worse safety record than their corporate counterparts do.
back to top 
For years the standard operating procedure for the medical device world has been: Bring good tech to patients and be rewarded by the market. Healthcare reform and particularly the consolidation of doctor practices is making this model obsolete. Now the challenge is more along the lines of “Justify your existence.”
Device companies have to revamp the traditional business model to show how their products save money and help patients in the long term.
Image: http://medcitynews.com
back to top 
NEW YORK (Reuters) – Ditching handshakes in favor of more informal fist bumps could help cut down on the spread of bacteria and illnesses, according to a study released on Monday.
The study in the American Journal of Infection Control found that fist bumps, where two people briefly press the top of their closed fists together, transferred about 90 percent less bacteria than handshakes.
Image: By The U.S. Army (Fist bump) [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons
back to top 

We all struggle for a moment of insight. Whether you’ve been banging your head against the wall for days or just woke up to a problem this morning, the desire for a creative boost is a powerful one. It’s the same feeling that plagues writers facing a blank page or advertisers developing a brand’s next campaign—and learning how to achieve it can have a profound impact. Here are some tips to avoid your next headache or create something the world has never seen before:
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 13
Germantown Innovation Center

August 14
1776

August 14
Smokey Glen Farm Barbequers

September 10-12
Washington, DC | San Francisco, CA

September 15-16
Sheraton Pentagon City
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

The implications of the world’s largest collaborative biological project in the year 2000—the Human Genome Project-combined with the Big Data revolution in the medical sciences holds out tremendous promise for human health. Recognizing the imminent need for the next generation of bioinformatics and genomics tools, four academic entrepreneurs from the prestigious Indian Institute of Science co-founded Strand Genomics Inc. (Strand). “Our focus is on personalized clinical genomics and molecular diagnostics. To address the grand challenge of the “$1000 genome and million dollar interpretation”, we have developed a clinical genomics interpretation and reporting platform, StrandOmics,” says Vijay Chandru, Ph.D, Co-founder, Chairman and CEO, Strand.
back to top 
 Qiagen has acquired an exclusive global license from the University of Tokyo for the biomarker SF3B1. The company said it sees potential for developing companion diagnostics to guide myelodysplastic syndromes (MDS) treatment with new anticancer compounds under development that target the SF3B1 gene. Qiagen has acquired an exclusive global license from the University of Tokyo for the biomarker SF3B1. The company said it sees potential for developing companion diagnostics to guide myelodysplastic syndromes (MDS) treatment with new anticancer compounds under development that target the SF3B1 gene.
back to top 
 Inside sources reveal that Indian pharmaceutical generics firm Lupin, along with other U.S. pharma firms, is considering bidding for GlaxoSmithKline’s auctioned mature and generic drugs. Inside sources reveal that Indian pharmaceutical generics firm Lupin, along with other U.S. pharma firms, is considering bidding for GlaxoSmithKline’s auctioned mature and generic drugs.
GSK seeks to divest its lineup of mature drugs in an effort to improve its growth profile by getting rid of its off-patent drugs in North America and Western Europe. The drugs are estimated to bring in annual sales of 1 billion pounds and are expected to fetch two to three times the price on the auction block.
back to top 

GSK has submitted an application for the world’s first malaria vaccine to the European Medicines Agency (EMA).
The vaccine candidate, RTS,S, is produced in yeast cells and targets the Plasmodium falciparum malaria parasite, most common in sub-Saharan Africa. It uses GSK’s proprietary AS01 adjuvant system, containing QS-21 Stimulon adjuvant licensed from Antigenics, a subsidiary of Agenus, as well as monophosphoryl lipid A (MPL) and liposomes.
back to top 

Pfizer’s bid to get AstraZeneca has turned out to have a silver lining.
Dr Jane Osbourn, who runs MedImmune’s operations at Granta Park, told me: “It has gelled the team together at AstraZeneca and MedImmune (which is owned by AZ), and this is a silver lining to what has happened.
back to top 

Out of the roughly 2 million Americans suffering from degenerative heart disease, only about 50,000 of them receive life-saving mitral valve surgery. This is primarily because the most common treatment, an open-heart procedure, can last between three and six hours and is extremely complex and high-risk.
Maryland-based Harpoon Medical plans to revolutionize that process with a new device that could reduce the procedure to just 60 minutes. Using their device, a patient’s chest would remain closed and the heart would continue beating during the procedure. It would cut recovery time from weeks to days and reduce risk factors significantly.
back to top 

Advaxis, Inc. ADXS +2.08% , a clinical-stage biotechnology company developing cancer immunotherapies, has entered into a clinical trial collaboration with MedImmune, the global biologics research and development arm of AstraZeneca. The Phase I/II immunotherapy study will evaluate the safety and efficacy of MedImmune’s investigational anti-PD-L1 immune checkpoint inhibitor, MEDI4736, in combination with Advaxis’ lead cancer immunotherapy vaccine, ADXS-HPV, as a treatment for patients with advanced, recurrent or refractory human papillomavirus (HPV)-associated cervical cancer and HPV-associated head and neck cancer.
Both MEDI4736 and ADXS-HPV are cancer immunotherapies, a new class of treatments that use the body’s own immune system to help fight cancer. MEDI4736 is designed to counter the tumour’s immune-evading tactics by blocking a signal that helps tumours avoid detection, while ADXS-HPV enhances the ability of immune cells to combat the tumour. Preclinical evidence suggests that the combination of ADXS-HPV with a checkpoint inhibitor, such as MEDI4736, can enhance overall anti-tumour response.
back to top 

Holy Cross Germantown Hospital is moving closer to opening, forming an inaugural medical staff and ramping up orientation for those new hires, officials said Tuesday.
When the hospital opens Oct. 1, it will have about 580 people working on site, said spokeswoman Yolanda Gaskins. Already, a few Holy Cross Germantown hospital senior managers have begun working at the location using on-site trailers as offices, she said. The $202 million, 93-bed hospital on the campus of Montgomery College in Germantown will be the first new Montgomery County hospital in 35 years.
back to top 
 Montgomery County plans to launch a major technology initiative in its public schools in August, providing 40,000 laptops and tablets to students as part of a project that will expand quickly in coming years, officials said Thursday. Montgomery County plans to launch a major technology initiative in its public schools in August, providing 40,000 laptops and tablets to students as part of a project that will expand quickly in coming years, officials said Thursday.
back to top 
 Infectious diseases, pathogens and bioterrorism agents are all inside a new building downtown. Jessica Kartalija explains it’s great news for researchers at Johns Hopkins. It’s the newest addition to the Baltimore Science and Technology Park at Johns Hopkins. Inside, it’s a state-of-the-art lab that will keep Maryland on the cutting edge of public health and technology. Infectious diseases, pathogens and bioterrorism agents are all inside a new building downtown. Jessica Kartalija explains it’s great news for researchers at Johns Hopkins. It’s the newest addition to the Baltimore Science and Technology Park at Johns Hopkins. Inside, it’s a state-of-the-art lab that will keep Maryland on the cutting edge of public health and technology.
back to top 
 The Intellectual Property Owners (IPO) recently published a list of the top 100 universities worldwide granted U.S. patents in 2013, and among them were three local schools: Johns Hopkins University, the University of Maryland and the University of Virginia. The Intellectual Property Owners (IPO) recently published a list of the top 100 universities worldwide granted U.S. patents in 2013, and among them were three local schools: Johns Hopkins University, the University of Maryland and the University of Virginia.
back to top 

Apollo Hospitals today announced a partnership with Strand Life Sciences to launch the latter’s genomic tests across its hospitals.
The partnership in gene-based diagnostics with the Bangalore-based healthcare research company will help doctors at Apollo to develop personalised medicine for targeted treatments and better patient outcomes.
back to top 

The Johns Hopkins University has joined the National Science Foundation’s National Innovation Network and becomes the fourth member university in the NSF Innovation Corps regional collaboration led by the University of Maryland, along with the George Washington University and Virginia Tech.
The NSF has approved a request from the three original universities to officially include Johns Hopkins in the I-Corps program’s “node” in the Mid-Atlantic called DC I-Corps, which was formed last year with $3.75 million in NSF funding.
back to top 

August 13, 2014 3:30 – 5:00 PM
Germantown Innovation Center, 20271 Goldenrod Lane, 2nd Floor, Germantown, MD 20874
PRESENTERS: Konstantina Manjoros Katcheves, Esq., Vice President and Global Head of Intellectual Property, Lonza Group, Ltd.
ABSTRACT:
Lonza Group Ltd., headquartered in Basel, Switzerland, is a worldwide leader in supplying the pharmaceutical and biotechnology industries with biopharmaceuticals. Come to learn first-hand how you can partner and collaborate with this international firm and develop a win-win relationship with Lonza.
Biography:
Konstantina Manjoros Katcheves, Esq. As global head of the intellectual property department at Lonza, Tina is responsible for patent, trademark and licensing strategy as well as related litigation. She manages a worldwide team of professionals for the Basel, Switzerland-based pharmaceutical company. She is responsible for intellectual property strategy and business alignment including patent, trademark and licensing of Lonza IP for Lonza’s Biologics businesses, including cell therapy and antibody therapeutics.
Tina has past experience in private practice, as a USPTO examinerand registered patent attorney. She is an author and speaker on a wide variety of topics including intellectual property and commercialization.
back to top 

Eight startup companies are among 67 recipients of Maryland Innovation Initiative awards in fiscal 2014.
The Maryland Innovation Initiative gave a total of $6.4 million to research projects and startups with ties to universities in the state. The innovation initiative is designed to spur commercialization of university research.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
- Request for Information: Shared Instrumentation Grant Program (S10)
(NOT-OD-14-104) Division of Program Coordination, Planning and Strategic Initiatives, Office of Research Infrastructure Programs
- Extramural Loan Repayment Program for Clinical Researchers (LRP-CR)
(NOT-OD-14-105) National Institutes of Health
- Extramural Clinical Research Loan Repayment Program for Clinical Researchers from Disadvantaged Backgrounds (LRP-IDB)
(NOT-OD-14-106) National Institutes of Health
- Extramural Loan Repayment Program for Pediatric Research (LRP-PR)
(NOT-OD-14-107) National Institutes of Health
- Extramural Loan Repayment Program for Contraception and Infertility Research (LRP-CIR)
(NOT-OD-14-108) National Institutes of Health
- Extramural Loan Repayment Program for Health Disparities Research (LRP-HDR)
(NOT-OD-14-109) National Institutes of Health
- Notice of the National Institute on Deafness and Other Communication Disorders (NIDCD) Participation in PA-14-114 “Behavioral Interventions to Address Multiple Chronic Health Conditions in Primary Care (R01)”
(NOT-DC-14-003) National Institute on Deafness and Other Communication Disorders
- Notice of Change to the Award Budget and Submission Requirements for PAR-12-198 “Improving Diet and Physical Activity Assessment (R01)”
(NOT-DK-14-023) National Institute of Diabetes and Digestive and Kidney Diseases
- Notice of NICHDs Participation in PAR-13-055 “Dissemination and Implementation Research in Health (R01)”
(NOT-HD-14-017) Eunice Kennedy Shriver National Institute of Child Health and Human Development
- Request for Information: NHLBI Whole Genome Sequencing Project (NHLBI-WGS)
(NOT-HL-14-030) National Heart, Lung, and Blood Institute
- Notice of the Change in the Expiration Date for PA-11-347 “NINDS SBIR Technology Transfer (SBIR-TT [R43/R44])”
(NOT-NS-14-038) National Institute of Neurological Disorders and Stroke
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 
 Jennifer Hammaker has watched biotech company respEQ Inc. grow from an experiment in a Johns Hopkins lab to a fledgling startup now looking for investors. Jennifer Hammaker has watched biotech company respEQ Inc. grow from an experiment in a Johns Hopkins lab to a fledgling startup now looking for investors.
RespEQ is a prime example of what Hammaker, who leads the Maryland Innovation Initiative, hopes the state’s research commercialization program will produce more of in the future.
back to top 
 The startup world will perk up to hear that RockthePost and CoFoundersLab recently merged to form Onevest. Together the two companies will provide a single source for startup founders to not only find investors but also find the right co-founders all online. The startup world will perk up to hear that RockthePost and CoFoundersLab recently merged to form Onevest. Together the two companies will provide a single source for startup founders to not only find investors but also find the right co-founders all online.
back to top 
 Kaiser is well known healthcare delivery model and outcomes, but, it turns out, it’s also among the best at engaging members via the web and mobile apps. The organization says in its 2013 annual report (just released) that about 4.4 million of its 9.1 million members use its online health management platform My Health Manager. Kaiser is well known healthcare delivery model and outcomes, but, it turns out, it’s also among the best at engaging members via the web and mobile apps. The organization says in its 2013 annual report (just released) that about 4.4 million of its 9.1 million members use its online health management platform My Health Manager.
back to top 
 It helps to have a friend. And for several pharma giants scrambling to cut R&D costs, those friends are venture capital firms that co-invest in startups whose new drugs and platform technologies appear attractive. In some cases, these pharma-venture “alliances” launch the startups, using the expertise of VC firm partners and company executives. It helps to have a friend. And for several pharma giants scrambling to cut R&D costs, those friends are venture capital firms that co-invest in startups whose new drugs and platform technologies appear attractive. In some cases, these pharma-venture “alliances” launch the startups, using the expertise of VC firm partners and company executives.
back to top 

He’s pointing out a challenge for entrepreneurs, not griping. He’s lived in California, Boston and New York, and he chose this area for a reason — “Maryland-D.C. is the mecca of security.”
So far, the bet’s paid off. ZeroFOX, which focuses on cybersecurity threats from social media, has raised $13 million from both local and out-of-state investors. Among them is Sourcefire’s former chief financial officer. That’s allowed the company to expand from its original two people to nearly 50.
back to top 

Like its business incubators for technology, cybersecurity and biotech, Montgomery County hopes a kitchen incubator will help food entrepreneurs thrive in the county.
The county is seeking proposals for a private partner to help “accelerate the commercialization of creations from local food entrepreneurs” by establishing a kitchen incubator, a shared kitchen space food entrepreneurs can use to start their businesses.
back to top 
There is clearly something wrong with pharmaceutical innovation.
Antibiotic-resistant infections sicken more than two million Americans every year and kill at least 23,000. The World Health Organization has warned that a “post-antibiotic era” may be upon us, when “common infections and minor injuries can kill.” Even the world’s tycoons consider the proliferation of antibiotic-resistant bacteria one of the crucial global risks of our times, according to a survey by the World Economic Forum.
back to top 

NextNav LLC, the bicoastal location services venture launched by XM Satellite Radio founder Gary Parsons, announced on Thursday a $70 million Series D funding led by New Enterprise Associates and Oak Investment Partners.
The eight-figure round is the latest high-dollar financing for NextNav, which two years ago reported a $50 million raise from Columbia Capital. Columbia also joined in the most recent round, alongside Telcom Ventures and Goldman Sachs Investment Partners.
back to top 

On Thursday, July 17, four science experts served as witnesses at the U.S. Senate Committee on Commerce, Science, and Transportation hearing, “The Federal Research Portfolio: Capitalizing on Investments in R&D.” The hearing considered the federal government’s role in research and development (R&D), and the nation’s STEM education and outreach initiatives.
Attendees in the Capitol hearing room were Mariette DiChristina, editor in chief and senior vice president of Scientific American; Vinton G. Cerf, computer scientist, Google’s Internet Evangelist and one of the fathers of the Internet; Neal F. Lane, former director of the White House Office of Science and Technology Policy; and Stephen E. Fienberg, professor of statistics and social science at Carnegie Mellon University.
back to top 

Maryland companies raised $64 million in venture capital funding this spring, with some of the biggest payouts flowing to Baltimore cybersecurity startups.
That’s according to the latest MoneyTree report from PricewaterhouseCoopers and the National Venture Capital Association. The report, which uses Thomson Reuters data, tracks money flowing to startups and later-stage firms across the country.
back to top 
 Founders Fund already stands out for backing startups with radical ideas, including Counsyl, Hampton Creek, Planet Labs, and SpaceX. Now the venture firm is making a formal commitment to funding startups in aeronautics, life sciences, nanotechnology, and other geeky realms. Founders Fund has dedicated a small percentage of its new $1 billion fund to a new initiative called FF Science, through which the firm will make seed-stage deals. Founders Fund already stands out for backing startups with radical ideas, including Counsyl, Hampton Creek, Planet Labs, and SpaceX. Now the venture firm is making a formal commitment to funding startups in aeronautics, life sciences, nanotechnology, and other geeky realms. Founders Fund has dedicated a small percentage of its new $1 billion fund to a new initiative called FF Science, through which the firm will make seed-stage deals.
back to top 
 While considerable attention has been heaped on the public health insurance exchanges over the year, private health insurance exchanges “are experiencing hyper-growth” and enrollment could exceed that of public exchanges by 2017, “if not sooner,” according to Accenture. While considerable attention has been heaped on the public health insurance exchanges over the year, private health insurance exchanges “are experiencing hyper-growth” and enrollment could exceed that of public exchanges by 2017, “if not sooner,” according to Accenture.
back to top 

After decades as a technological laggard, medicine has entered its data age. Mobile technologies, sensors, genome sequencing, and advances in analytic software now make it possible to capture vast amounts of information about our individual makeup and the environment around us. The sum of this information could transform medicine, turning a field aimed at treating the average patient into one that’s customized to each person while shifting more control and responsibility from doctors to patients.
The question is: can big data make health care better?
back to top 

Capital efficiency has become a mantra at Atlas, one shared by a number of other early stage biotech investors. It’s a term often repeated in discussions about building young companies, and yet it has become clear that there’s no consistent definition of what the term means.
A common perception is that capital efficiency is synonymous with tightly constrained, small amounts of investor capital – the “small ball” criticism of capital efficiency. Others think it means only ultra-lean, asset-centric, virtual companies. There’s also the perception that you can’t build something big if you are capital efficient.
back to top 
 In 1957, eight entrepreneurs decided to do something that seemed crazy. They launched a new tech company called Fairchild Semiconductor in a small town south of San Francisco. The entrepreneurs had a difficult start, but Fairchild eventually became the first major computer chip company in the region. In 1957, eight entrepreneurs decided to do something that seemed crazy. They launched a new tech company called Fairchild Semiconductor in a small town south of San Francisco. The entrepreneurs had a difficult start, but Fairchild eventually became the first major computer chip company in the region.
back to top 

The BioMaryland Center today joined with Medicen Paris Region to announce a co-funded international project to support commercialization and collaboration in their two regions. Opticul Diagnostics of Rockville, Md. and Diafir of Rennes, France have been selected as the first two participants of a partnership that was created at BIO International 2012 to promote collaboration on products to improve and speed diagnoses and patient care. The announcement was made at the BIO International Convention 2014 in San Diego.
This is the first jointly funded international project undertaken by the BioMaryland Center, which is part of the Department of Business and Economic Development (DBED) of Maryland. The state of Maryland is the fourth largest biopharma concentration in the United States and Medicen Paris Region represents one of Europe’s largest clusters of life science and health care companies.
back to top 

It’s not unusual for hospitals to have innovation centers these days, but the folks at New York Presbyterian Hospital have chosen an interesting satellite office for theirs: in the offices that house health IT accelerator Blueprint Health. It’s a remarkable development for an industry in which hospitals have tended to hold startups at arm’s length.
Although hospitals and health systems often welcome entrepreneurs’ motivation to improve healthcare, their knack for identifying problems and developing solutions as well as using technology to speed up care delivery, the complexities of making startup solutions work in healthcare has been challenging. Providers are frequently frustrated by an insufficient understanding by startups of hospital workflows, the complexities of implementing new technology along with the safety and regulatory rigors involved. On the flipside, healthcare startups are often frustrated by what they perceive as the snail-like pace of hospitals to adopt new health IT tools and make care delivery more efficient.
back to top 

More than 80% of U.S. consumers conduct online research prior to making everyday purchases. This is a statistic that is growing, and extends beyond products to include services and, finally, even healthcare. With the abundance of information available, consumers are empowered–they want to be as informed as possible when selecting physicians, but how does someone navigate through the overwhelming results of Google searches and decide how to best make a decision as important as selecting a healthcare provider? I think most people would agree that selecting a physician and selecting where to have dinner have highly differing degrees of importance when it comes to online research.
back to top 

There’s a panoply of alternative funding streams for healthcare startups out there – fledgling companies are seeking out avenues like prepaid revenue models, grant funding in lieu of venture capital, and the ever-popular crowdfunding approach.
The outcomes can be a mixed bag, however, said a panel of speakers at MedCity News’ CONVERGE conference in Philadelphia today.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
| | | | | | | | | |