|
|
|

BioHealth Innovation clients Alex Lai (Speed BioSystems), Matthew Mulvey (Benevir), David Cetlin (MockV Solutions), and BHI Entrepreneur-in-Resident Ram Aiyar meet with Bahija Jallal.
Dr. Bahija Jallal is Executive Vice President, MedImmune, responsible for biologics research, development and clinical activities. As part of AstraZeneca’s Senior Executive Team, Bahija is tasked with advancing the biologic organisation’s pipeline of drugs targeting cancer, infections, respiratory and inflammatory diseases, cardiovascular and gastrointestinal disorders and pain. (Astrazenica Bio)
back to top 

Biopharmaceutical company Emergent BioSolutions could begin manufacturing an experimental Ebola drug at its Baltimore facility.
Rockville-based Emergent is one of three advanced laboratories asked by the Biomedical Advanced Research and Development Authority (BARDA) to submit a plan for manufacturing ZMapp. The drug has been used among infected health workers in Africa but supplies have run out. BARDA will select one or more of the labs to make more of the drug.
back to top 
 Summary of Position: Summary of Position:
The Director, Regulatory Affairs is the regulatory representative on the Product Development Teams providing regulatory support and advice. The Director will facilitate regulatory strategy development and implementation, take the lead on regulatory submissions, and author, review, and coordinate quality submissions to regulatory agencies. The Director will prepare for meetings, teleconferences, and other communications with FDA, educate teams regarding regulatory risks and implications for strategy and product development activities, and utilize technical knowledge and effectively apply regulations and guidelines to the product development process.
back to top 
Join us in welcoming two new interns to our team!
|
Vanessa Sorto
Accounting and Human Resources Intern
Vanessa Sorto joined BHI on September 2014 as an accounting and human resources intern. She is responsible for supporting the director of finance and human resources in assembling monthly expense reports for BHI senior staff, reconciling bank accounts, preparing monthly financial reports, assisting in audit preparation, and maintaining payroll. She is currently pursuing a bachelor’s degree at The University of Maryland, College Park in Accounting and Information Systems. She is a Hillman Entrepreneur scholarship recipient.
|
 |
|
Kurt Herzog
Business & Research Analyst
Kurt Herzog joined BHI in 2014 as a Business & Research Analyst. He assists BHI’s client companies with market entry strategies for their products by engaging with clients to understand current products, researching existing and potential markets, and analyzing business strategy. His work includes developing executive summaries, investor slide decks, tailored presentation materials, and creating financial models for presentation to investors.
Kurt also works with Tania Fernandez on the BioHealth Gap Fund, a $50 million fund announced October 2014 in partnership with the EAGB to support early-stage therapeutics, medical device, diagnostics, and health IT companies in central Maryland. Kurt has experience in investment banking, international micro-lending, renewable energy project finance and project development, forest carbon sequestration monitoring, institutional investing in hedge funds, and working for a non-profit startup accelerator. Kurt holds a BA in Economics and Environmental Studies with a Natural Science concentration from Bowdoin College. He also completed substantial coursework in chemistry.
|
 |
back to top 

Don’t miss the third annual full-day Innovation 2 Commercialization (I2C) conference featuring three informative plenary sessions to help launch, commercialize and fund YOUR technology to make your business venture profitable!
Hear panels on innovation, commercialization and financing featuring speakers from MedImmune, Brain Sentry, Naval Research Lab and more. Plus, learn about the “Amplimmune Story” and enjoy lunch as you discuss issues affecting your innovation or small business with a subject matter expert from a federal lab, university tech transfer office, venture capital firm, business service organization and others who serve as your table host.
back to top 

Join the BioBuzz networking group at our next free event. Along with 100’s of the region’s bioscience workers, you too could be making new connections, getting jobs and helping to build a stronger, more connected industry through the BioBuzz community!
The event is always free and we offer free drinks to the first 50 to 100 who arrive depending on how much we’ve been sponsored on a given month. People from all around the the region are coming out each month for this unique and welcoming monthly happy hour. It’s a great place to meet up with coworkers past and present, make new connections or just catch up on the latest industry gossip If you’re new and haven’t yet made it out to an event, then we hope that you’ll join us this month and see what all the Buzz is all about.
back to top 

One of the two Ebola-infected Dallas nurses was admitted to a National Institutes of Health biocontainment unit in Bethesda on Thursday, just as researchers at the University of Maryland School of Medicine in Baltimore are beginning the first human trials of an Ebola vaccine in Africa.
Dr. Myron Levine, a researcher at the medical school, is a member of the international medical group leading the efforts to end the outbreak. Ebola has infected more than 8,000 people since the first reported case in March, according to the Centers for Disease Control and Prevention.
back to top 

Applications are now open for DreamIt Health’s second Baltimore cohort.
DreamIt Ventures will accept applications through Dec. 5, according to a DreamIt blog post. The first cohort last year included startups like Protenus, Aegle and Quantified Care.
back to top 

ASTRAZENECA has announced a four-way collaboration which will strengthen the pharmaceutical giant’s link with Cambridge University.
The firm, together with its biologics research and development arm MedImmune, has agreed the new collaboration as it prepares to move its global headquarters to the city. It builds on an existing strategic partnership between the organisations.
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association for life science and technology, has been awarded a three-year, $225,000 federal grant designed to help job seekers gain skills in the growing fields of cyber technology and cyber security.
The grant is part of a $15 million federal investment in the Cyber-Technology Pathways Across Maryland (C-PAM) Consortium, spearheaded by Montgomery College. The consortium, which is comprised of 14 community colleges and trade associations, aims to prepare women and other underrepresented populations for jobs in the rapidly growing cyber industry.
back to top 

Sucampo Pharmaceuticals has announced that it signed an amendment to the existing collaboration and license agreement (Collaboration Agreement) covering the United States and Canada for AMITIZA(R) (lubiprostone) with Takeda Pharmaceutical Company Ltd. The amendment includes various modifications to the Collaboration Agreement including the extension of the current term, minimum commercial investment during the current term and various governance changes allowing Takeda additional flexibility in commercializing AMITIZA.
During the extended term, which will begin on January 1, 2021, Takeda will split with Sucampo the gross profits of branded AMITIZA for any dosage strength and form for the existing indications in the U.S. and Canada. In addition, on April 1, 2015 Takeda will no longer reimburse Sucampo for the product details made by Sucampo sales representatives to healthcare professionals as well as other ancillary costs of the sales force.
back to top 

As the tech industry falls back in love with urban America, Joel Marcus, landlord to the booming biotechnology business, is perfectly positioned to strike it rich.
In Manhattan, overlooking the East River and sandwiched between New York University Langone Medical Center and Bellevue Hospital, two gleaming 16-story towers act as beacons to the booming biotech sector.
Completed in 2010, the 310,000-square-foot Alexandria Center for Life Science is already 100% occupied. It contains a hub for Eli Lilly’s cancer business and a Pfizer PFE -1.69% lab dedicated to exploring leads generated by academic researchers. The second building, some 410,000 square feet of labs and office space, was finished in January and is already 60% full. Roche, the anchor tenant, says moving 250 clinical trial specialists there from Nutley, N.J. has resulted in 25 new collaborations with charities, biotechs and New York hospitals. There’s also an accelerator for startups, an enviro-friendly garden and fancy restaurants to feed all those scientists.
back to top 

Becton Dickinson (NYSE: BDX) has acquired GenCell Biosystems, a privately-held Irish biotech company that has developed proprietary technologies that address key biological analysis protocols – from library preparation of Next Generation Sequencing (NGS) to genotyping for agricultural applications.
“We are excited with the GenCell Biosystems acquisition as it provides BD entry into the Next Generation Sequencing market, a fast-growing segment with the potential to have a significant impact on healthcare,” said Linda Tharby, Group President, BD. “The acquisition gives BD access to the NGS market with a differentiated platform that will provide a base to further grow our genomics offerings.”
back to top 

QIAGEN N.V. (NASDAQ: QGEN)(Frankfurt Prime Standard: QIA) announced today that its business area, QIAGEN Bioinformatics, has been named by Genomics England (GeL) an assessment winner for the UK100K project, along with other life science technology providers. The company will be providing project researchers access to Ingenuity® Variant Analysis™, a powerful web-application that allows users to rapidly identify and prioritize disease causing genetic variants using advanced analytics powered by published biological evidence and large numbers of sequenced genomes.
back to top 

Bicycle’s founding venture capitalist (VC), Atlas Ventures joined forces this month with SV Life Sciences and three corporate VCs – Novartis (NVS) Venture Fund, GlaxoSmithKline’s (GSK) SR One and Astellas Venture Management to invest $32m (€25m) in the Cambridge-based biotech company.
Bicycle Therapeutics was established in 2009 as a spin-off of the Medical Research Council Laboratory of Molecular Biology (Cambridge), based on the work of the scientific founders Sir Gregory Winter and Professor Christian Heinis from Trinity College, Cambridge. Winter, a renowned scientist in the biotech industry, also founded Domantis and Cambridge Antibody Technology. Founded in the 80s, Cambridge Antibody Technology was a pioneer in British biotechnology, and was listed on the UK stock exchange in 1997, raising £43m. In 2006, AstraZeneca bought the company.
back to top 

A $5 million Innovation Challenge Fund (ICF), to encourage and support academic groups and small companies in collaborative efforts in the development of bioelectronic medicines, has been announced by GSK. This funding programme is in addition to the company’s prior commitment of a $1 million award (December 2013), for the team that first solves the GSK Bioelectronics Challenge.
Bioelectronic medicine is focused on producing miniaturised, implantable devices that could be programmed to read and correct electrical signals passing along the nerves of the body, to treat disorders as diverse as inflammatory bowel disease, arthritis, asthma, hypertension and diabetes. The ICF funded work and the Innovation Challenge’s winning entry will be made freely available to the global research community.
back to top 

Venture capital investments in Maryland are still working their way back up, according to a report released this week by PricewaterhouseCoopers and the National Venture Capital Association.
In the third quarter of 2014, venture capitalists spent $89 million, up 34 percent from the second quarter’s $66.5 million, but far off the third quarter number from last year, which hit $142 million, according to the report.
back to top 
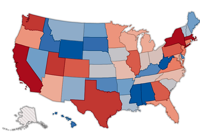
In the Boston-versus-New York rivalry, the Red Sox and the Yankees were also-rans this baseball season. Now, Massachusetts and New York are in another battle for the No. 2 spot: U.S. venture capital investment.
New York is leading Massachusetts in total venture capital invested so far this year, which if it held up would be the first time the Empire State edged out its East Coast rival and took second place behind industry leader California since at least 1992.
back to top 

A BIOTECHNOLOGY company founded by Limerick man Kieran Curran has been snapped up for $150m by a US firm.
GenCell Biosystems, a company based in Raheen with offices in the US, was acquired late last week by Becton Dickinson, a company listed on the New York Stock Exchange.
back to top 
ATCC, the premier global biological materials resource and standards organization, announces the release of ATCC® Minis to support quality control (QC) testing in pharmaceutical and industrial labs, during the PDA 9th Annual Global Conference on Pharmaceutical Microbiology in Bethesda, MD, Booth # 304.
Healthcare, personal care product, and cosmetic manufacturers are required to test the bio-burden and sterility of their products and production environments to ensure consumer safety. Global alignment and harmonization of microbial testing requirements among the United States Pharmacopeia (USP), Japanese Pharmacopeia (JP), and European Pharmacopeia (EP), have resulted in the need for consistent and reliable control organisms at less than five passages from the ATCC reference stock for reproducible results.
back to top 

A jaw-dropping report released by the World Health Organization on October 14, 2014 reveals that 1 in 20 Ebola infections has an incubation period longer than the 21 days which has been repeatedly claimed by the U.S. Centers for Disease Control.
This may be the single most important — and blatantly honest — research report released by any official body since the beginning of the Ebola outbreak. The WHO’s “Ebola situation assessment” report, found here, explains that only 95% of Ebola infections experience incubation within the widely-reported 21-day period.
back to top 

I joined J&J Consumer Companies about four years ago to start its Digital Center of Excellence. Our role initially was to build capabilities and develop strategy that served multiple brands in multiple regions, so I did a landscape overview to help develop the approach. What I saw was that we had hundreds of different websites and digital platforms that we were operating upon globally. If you want to get a message across globally on your owned assets, you need to do that in the same way across the world.
back to top 

The Center for Innovative Technology (CIT) announced this week the release of the Commonwealth Research Commercialization Fund (CRCF) annual report for FY2014, showing growth in new patents, products and innovative companies.
Karen Jackson, Virginia’s Secretary of Technology, said, “CRCF plays an important role in the acceleration of innovation in the Commonwealth by funding essential research and commercialization projects. The investments we make in research commercialization plant the seeds that are growing the New Virginia Economy.”
back to top 

Bioinformatics giant Illumina (NASDAQ: ILMN) is getting into the accelerator game, along with other players in the life sciences and other fields. On Wednesday it announced the first three startups chosen to start the program this fall at its San Francisco lab space.
San Diego-based Illumina, which makes genomic analysis systems, unveiled the program in February, joining the growing list of academic institutions, venture-backed groups, and life science corporations building start-up space in the Bay Area and beyond. Not least among the benefits of the Illumina accelerator program is access to the company’s high-end gene sequencing systems, which are installed in the lab space Illumina has leased near the Mission Bay campus of the University of California, San Francisco.
back to top 

The Eastern Shore Entrepreneurship Center (ESEC), Rural Maryland Council (RMC), and Maryland Capital Entreprises (MCE) have joined together to offer the third annual Eastern Shore Business Competition. All three organizations strive to advance and expand the entrepreneurial ecosystem on Maryland’s Eastern Shore, as well as throughout Maryland, and collaborate to execute a business competition that will draw greater attention to the Eastern Shore and attract entrepreneurs to the opportunities and resources available in the region. The application process for the competition has begun and we are looking forward to your submissions!
The purpose of the competition is to promote and encourage the startup of new businesses and in turn the creation of new jobs on Maryland’s Eastern Shore.
back to top 

Ideas are the juice that powers our economy with innovation happening fast on multiple technology fronts. Rapid developments are in play in areas as diverse as 3D printing, Ultra HD, sensors, health care, automotive electronics, agriculture, transportation, biotech and genetic mapping.
The $211 billion consumer electronics (CE) industry is at the vanguard of innovation. Just last year, the U.S. Patent Office issued a record 277,835 patents. We are at the beginning of a surge of technology advances that we will all benefit from.
back to top 

On a massive bus in the heart of America, a group of tech entrepreneurs and investors are spreading glad tidings about entrepreneurship and startups in cities that don’t exactly scream innovation. Many people have tried to jumpstart the innovation engine across the country, but few have as much experience and clout to pull it off as Steve Case, co-founder of AOL, and chairman of DC-based investment firm Revolution.
back to top 

Chairs: we sit in them, work in them, shop in them, eat in them and date in them. Americans sit for most of their waking hours, 13 hours every day on average. Yet chairs are lethal.
This grim conclusion may surprise you, but 18 studies reported during the past 16 years, covering 800,000 people overall, back it up. In 2010, for example, the journal Circulation published an investigation following 8,800 adults for seven years.
back to top 

The National Institutes of Health (NIH) has launched a pilot program to help life science entrepreneurs commercialize their technology, based on a course developed by the University of California, San Francisco. The course was first taught last fall by the Entrepreneurship Center at UCSF and Steve Blank, architect of the Lean LaunchPad framework. UCSF and Blank adapted the Lean LaunchPad methodology to be applicable for life science and healthcare ventures.
back to top 
Antibiotic resistance is taking its toll on the pharmaceutical industry: Drugs are getting retired from clinical circulation, because many are starting to get rendered ineffective, according to an article from Washington University of St. Louis. It highlights the work of WUSTL’s Michael Kinch, associated vice chancellor of its Center for Research Innovation in Business:
back to top 

University of Maryland, Baltimore’s $200 million proton therapy center will begin treating cancer patients in a year.
The center’s first of five treatment rooms will begin treating patients in October 2015. The entire building is expected to be complete in two years. The 110,000-square-foot building is part of the University of Maryland BioPark in Baltimore off Martin Luther King Boulevard.
back to top 

The Center for the Advancement of Science in Space (CASIS) has announced grant awards for three projects focused on enabling technologies from the International Space Station (ISS). These awards stem from the CASIS Request for Proposals (RFP) “Enabling Technology to Support Science in Space for Life on Earth.” CASIS is the nonprofit organization managing research onboard the ISS U.S. National Laboratory.
The purpose of this RFP was to identify and support technology development projects that would enable increased use of ISS for Earth benefits—for example, improvements in hardware/capabilities or methods to improve bandwidth, throughput, or quality of future research projects.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 22
American Tap Room

October 23
Universities at Shady Grove

October 28
British Embassy

November 5- 6
FLCVirtual

November 6
IBBR
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 Dutch biotech MIMETAS B.V. announces that it has raised 5.2 million dollar to fund its expansion as a global leader in organ-on-a-chip technology. Venture capital investors Zeeuws Investerings Fonds (ZIF) and Participatiemaatschapij Oost Nederland (PPM Oost) have joined forces with national and regional partners to fund development and sales of MIMETAS’ unique 3D disease- and tissue modelling technologies. Dutch biotech MIMETAS B.V. announces that it has raised 5.2 million dollar to fund its expansion as a global leader in organ-on-a-chip technology. Venture capital investors Zeeuws Investerings Fonds (ZIF) and Participatiemaatschapij Oost Nederland (PPM Oost) have joined forces with national and regional partners to fund development and sales of MIMETAS’ unique 3D disease- and tissue modelling technologies.
Jos Joore, co-founder and Chief Business Officer of MIMETAS, acknowledges that these resources arrive at a strategically important moment: “Collaborations with top-tier pharmaceutical companies are expanding rapidly, an example of this is the recently announced 1.6 million dollar kidney-on-a-chip project with Roche, Pfizer and GlaxoSmithKline. The funds will be used to scale up the production of OrganoPlates™ and strengthen our activities in predictive preclinical model development, thus consolidating our leadership in this highly competitive field.”
back to top 
 Wednesday, October 15, 2014, 08:30am – 11:45am Wednesday, October 15, 2014, 08:30am – 11:45am
Please join BioHealth Innovation and the National Institutes of Health in a session to learn more about the Small Business Innovation Research (SBIR) program, one of the largest sources of early-stage capital for innovative small companies in the United States. SBIRs are non-dilutive federal research and development grants and contracts that fund innovative research efforts intended for commercialization by small business
This free event brought to you by BioHealth Innovation allows you to hear directly from the largest NIH Institutes on what they are looking for in a high quality grant application. These Institutes include the National Cancer Institute (NCI), National Heart Lung & Blood Institute (NHLBI), National Institute of Allergy & Infectious Disease (NIAID), and the National Institute of Neurological Disorders & Stroke (NINDS).
After remarks from each Institute, we will break out into a speed networking session to talk about your project with program managers.
Contact Ethan Byler at ebyler@biohealthinnovation.org if you are interested in scheduling one-on-one meetings.
back to top 

Don’t miss the third annual full-day Innovation 2 Commercialization (I2C) conference featuring three informative plenary sessions to help launch, commercialize and fund YOUR technology to make your business venture profitable!
Hear panels on innovation, commercialization and financing featuring speakers from MedImmune, Brain Sentry, Naval Research Lab and more. Plus, learn about the “Amplimmune Story” and enjoy lunch as you discuss issues affecting your innovation or small business with a subject matter expert from a federal lab, university tech transfer office, venture capital firm, business service organization and others who serve as your table host.
back to top 

The patent covers novel MDCK cells that have been adapted to grow in serum-free culture medium as well as cultivation techniques MedImmune uses to increase vaccine production titres.
According to the patent the modified cells support replication of attenuated influenza virus to a log10 TCID50/mL of at least 7, which is a significant advance on previous highest viral titres – around 4 log10 TCID50/mL – described in the literature .
back to top 

Joel S. Marcus, founder, chairman, and chief executive of Alexandria Real Estate Equities, has focused the real estate investment trust on developing properties for the life sciences industry. While it operates in other biotechnology and medical technology hubs from New York to San Francisco, the Pasadena, Calif., company’s largest cluster of properties is in Greater Boston, where it owns 3.5 million square feet of space in Cambridge’s Kendall Square, along Route 128, and in Boston’s Longwood Medical Area. On a visit to Cambridge, the 67-year-old Marcus spoke with Globe reporter Robert Weisman. Here’s what he found out:
back to top 

As Alexandria Real Estate Equities (NYSE: ARE) marks the 20th anniversary of its founding, the provider of state-of-the-art real estate for the science and technology industries is seeing occupancy, demand and new development all reach record levels.
“It’s an interesting time because we’re seeing the innovation economy doing miraculously well,” said Joel Marcus, founder and CEO of Pasadena, Calif.-based Alexandria.
back to top 

MedImmune, the global biologics research and development arm of Anglo-Swedish drug major AstraZeneca,…
In order to access this content you need to be logged into the site and have an active subscription or trial subscription. Please login, take a free trial or subscribe in order to continue reading.
back to top 

Swiss drug major Roche (ROG: SIX) has acquired exclusive rights to a primer extension-based target enrichment technology and associated patent applications filled by therapeutic target discovery company AbVitro.
AbVitro and Roche scientists are to collaborate on the development and application of the technology, which will be used to support next-generation sequencing directly from blood or other biological samples, a key advantage for clinical sequencing applications. It will be incorporated into Roche’s sequencing unit research and development pipeline to support the strategy of providing a full next-generation sequencing workflow solution for clinical sequencing.
back to top 

Join the BioBuzz networking group at our next free event. Along with 100’s of the region’s bioscience workers, you too could be making new connections, getting jobs and helping to build a stronger, more connected industry through the BioBuzz community!
The event is always free and we offer free drinks to the first 50 to 100 who arrive depending on how much we’ve been sponsored on a given month. People from all around the the region are coming out each month for this unique and welcoming monthly happy hour. It’s a great place to meet up with coworkers past and present, make new connections or just catch up on the latest industry gossip If you’re new and haven’t yet made it out to an event, then we hope that you’ll join us this month and see what all the Buzz is all about.
back to top 

A Johns Hopkins neuroscientist hopes to fuel future research projects by turning his expertise into a video game that stars a dolphin.
Hopkins neuroscientist John Krakauer, who studies movement and human obsession with movement, worked with Hopkins software architects Omar Ahmad and Promit Roy, and Baltimore artist Kat McNally to create the game “I Am Dolphin.” The game was released through iTunes Oct. 9 and costs $2.99.
back to top 

The University Senate made progress on the policies and guidelines that address non-tenure-track faculty — or professional track faculty, as they will soon be addressed, after a proposal passed in the senate yesterday.
The proposal, which passed by a vote of 61–12 with four abstentions, aims to create an overall title for faculty who are not on the tenure track but contribute to the university through teaching, research or service.
back to top 

The National Institutes of Health on Thursday awarded almost $32-million in grants to more than two dozen institutions to devise innovative ways of helping researchers handle huge sets of data seen as increasingly central to future medical discoveries.
The grants are the first outlay in a project, announced last year and known as Big Data to Knowledge, that’s expected to involve more than $600-million in spending by 2020. Its goals include developing and distributing methods, software, and tools for sharing, analyzing, managing, and integrating data into medical research.
back to top 

In 2014, startups took home nearly $1M in cash and prizes from the InvestMaryland Challenge. For 2015 we have new categories and are racking up partner prizes like cash grants, incubator space, legal advice and consulting services.
Start your application today! Applications are due December 12th.
$100,000 Grand Prize Categories
- IT HARDWARE/SOFTWARE
Enterprise Software, Data Analytics, Social Media & Apps, E-Commerce and Marketing/ADTECH.
- DEFENSE & SECURITY
Cybersecurity, Unmanned Systems, Defense, Communications Infrastructure, Public Infrastructure and Grid Security.
- LIFE SCIENCES
Biopharmaceuticals, Medical Devices & Diagnostics, Nutraceuticals, Agriculture Bio & Aquaculture and Healthcare IT.
- SUSTAINABILTY & EXPLORATION
EdTech, Energy, Solar Power, Space & Satellite Technologies, Climate Change/Weather, Water Management and Materials Science.
back to top 

Nominations are now being accepted for the 2015 FLC Awards. Since its inception in 1984 the FLC Awards have become one of the most coveted honors in the technology transfer field, with over 200 Federal laboratories honored for their work in projects that advance the mission of technology transfer. To reflect the diversity in scope and number of technology transfer efforts undertaken by federal laboratories and their partners, seven categories of awards will be presented:
back to top 

A California-based business accelerator that considers applicants based on their test scores is considering launching a Baltimore chapter.
Founder Institute already operates in 61 cities, and now it’s looking for its first Baltimore class. Local organizers will be hosting events throughout the month of October to determine whether any local entrepreneurs meet their standards – talented folks who would be a part of the four-month program that would start later this year or toward the beginning of 2015.
back to top 

A business accelerator that admits people based on their entrepreneurial potential — not just their business idea — is looking for its first Baltimore class.
Founder Institute, a California-based organization that operates accelerator program in 61 cities, is considering launching a Baltimore chapter. Local organizers will be holding engagement events in October to gauge interest and determine if any local entrepreneurs are good candidates. The four-month program could start later this year or early next.
back to top 

The ETC (Emerging Technology Centers) www.etcbaltimore.com,Baltimore City’s award-winning technology innovation centers, announced today that The Abell Foundation will continue its support of AccelerateBaltimore™for 2015. Six companies will be selected for this intensive 13- week program and will be awarded $25,000 each to help propel their business ideas forward.
“We are absolutely thrilled that The Abell Foundation is continuing its support of AccelerateBaltimore (AB),” said Deborah Tillett, ETC’s president. “Of the 16 companies to successfully complete the program, 81% are still in business and have raised over $2.5 million in follow-on funding.”
back to top 

A pilot program launched this week in four NIH institutes looks to speed up development and commercialization of new products and services generated by projects funded through the agency’s Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) awards.
The first class of 21 three-person teams of researchers and entrepreneurs gathered in Chevy Chase, MD, this week, concluding Wednesday, for the launch of the NIH Innovation Corps (I-Corps™) Team Training Pilot Program. The teams are all based at therapeutics, diagnostics, and medical devices companies funded with NIH SBIR Phase I grants, awarded to establish feasibility of proof of concept for commercializable technology.
back to top 

For more than 3,000 years, traditional Chinese medicine was the predominant medical treatment in China. In recent decades, the practice has gained popularity in the the west.
Herbal medicines and various mind and body practices, such as acupuncture and tai chi have become a fashionable way to treat or prevent health problems.
An ancient practice finds its place in modern society. This is the Beijing Chinese Medicine clinic in Santa Monica, Los Angeles.
back to top 

T, business, human capital and other stakeholders can now use cloud-based analytics to more effectively attract, acquire, serve and engage customers, Deloitte Digital announced today. Deloitte Digital has created an analytics offering for Wave, the Salesforce Analytics Cloud that is designed to offer greater flexibility than on-premises solutions. Deloitte Digital’s approach to cloud-based analytics is designed to help companies create strategies for success in a new environment. An example of Deloitte Digital’s commitment to Salesforce Analytics Cloud is its Member Connect solution, a health care industry-focused accelerator with an intuitive approach to the customer journey focusing on omni-channel interactions and a holistic view of the customer.
back to top 
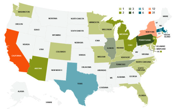
Of the 115 healthcare accelerators in the world, 87 are in the U.S., most of them are geared to digital health and are under two years old. Although many accelerator companies have created jobs and there have been some exits, most have yet to show anything for the investment in them. That’s understandable given the age of the average accelerator and given that it can take seven years before a company can realize a return on capital for investors. But the consensus of a new report published by the California HealthCare Foundation is that we should expect some consolidation soon.
back to top 

Downtown Partnership of Baltimore Inc. is launching a grant program aimed at getting more tech firms to consider moving downtown.
The nonprofit is offering six $10,000 grants to tech firms willing to give office space a try downtown for at least a year. Firms must locate to within Downtown Partnership’s “management authority area,” a 106-block area that includes the central business district, part of midtown’s west side and the west side of downtown up to Greene Street.
back to top 

While all The Seven Habits of Highly Effective People listed by Stephen Covey remain relevant and timeless, the one which resonates with me most is the seventh — sharpen the saw. In the life sciences industry, Covey’s concept of continuous improvement is more than just a habit, but a way of life. This is especially true for those who work in pharma and biopharma manufacturing — striving to maintain high quality, be on time with delivery, increase productivity (often with fewer resources) and so on. If you work in manufacturing, you are probably tempted to stop reading and get back to work.
back to top 

Health information technology (HIT) refers to a broad spectrum of technologies, ranging from personal health-monitoring applications to big data analytics. The venture capital firm Rock Health recently reported that venture capital funding in the HIT field reached $3 billion for 2014, well surpassing the $1.9 billion invested in the sector during 2013.[1] The LSN research team tracks investors in early stage life sciences, and we have noticed a growing interest in HIT as well.
back to top 

Mayor Vincent C. Gray, Interim Deputy Mayor for Planning and Economic Development M. Jeffrey Miller and DC Innovates today announced grants for eight tech startups through the Digital DC Tech Fund (DDCTF). DDCTF is a catalytic fund that provides grants of $25,000 to $200,000 along with a customized mentorship program for early- and growth-stage technology ventures in the District of Columbia.
“Each of these grantees represents a bright future for technology, innovation and economic growth in the District of Columbia,” Mayor Gray said. “Through the Digital DC Tech Fund, my administration is able to provide resources and opportunities that will allow grant recipients to grow their companies and continue to make the District a place where innovative companies can start, develop and thrive.”
back to top 

For creative writing, Joyce Carol Oates got it right when she advised, “Be daring, take on anything.”
But when you are trying to make a good first impression on you future boss, concision and confidence sets a qualified applicant apart from one who doesn’t sound sure of her own experience.
back to top 

Andrew Laver has long worked with life science startups through his work as an angel investor, venture capitalist and investment banking. Now he’s getting that perspective from one of Healthbox’s newest accelerators in Salt Lake City.
As I mentioned in another post, it’s interesting that an accelerator would take on so many medical device companies (four). But as Laver points out, it goes with the territory because there are so many in the region. Salt Lake City has the biggest concentration of medical device manufacturers in the country, according to Utah’s economic development corporation. It has more than 100 medical device companies.
back to top 

Failure to diagnose the Ebola case quickly in Texas raises questions about electronic health records, known as EHRs.
Information entered by a nurse was not transferred to doctors in an emergency room. With more of our medical records going digital, failures in the relatively new technology will become more important to all of us.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 15
Johns Hopkins University – Montgomery County

October 23
Universities at Shady Grove

October 28
British Embassy

November 5- 6
FLCVirtual

November 6
IBBR
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today that venture capitalist, Tania Fernandez, Ph.D., has joined the BHI team as a strategic advisor. Dr. Fernandez will be a member of the management team for a new BioHealth Gap Fund, which will provide up to $50 million in seed and early-stage equity investments to therapeutics, medical device, diagnostics, and health IT companies in Maryland. Additional BioHealth Gap Fund management team members include Richard Bendis, Ram Aiyar, Todd Chappell, and Ken Malone, who each bring domain knowledge and industry access to the fund.
“Dr. Fernandez has ten years of experience as a venture capitalist in the life sciences/biotechnology industry. Her work in Silicon Valley, along with her research experience at the National Cancer Institute, makes her a tremendous asset to the BHI team,” said Richard Bendis, BHI President & CEO. “Dr. Fernandez brings a West Coast investment perspective, and she will have an active role in helping to manage the BioHealth Gap Fund. She will also support our BHI Entrepreneurs-in-Residence and clients: helping our startups to grow and raise strategic funding.”
back to top 

The next generation of the nation’s only anthrax vaccine will be made in Lansing, an executive for Emergent BioSolutions Inc. said Tuesday.
There won’t be any new jobs as a direct result of the $29 million federal contract Emergent received earlier this month for production of the new drug, Adam Havey, president of the company’s biodefense division, said. But the new version of the vaccine will help keep current employees working.
back to top 

For the majority of drugs, end-of-lifecycle planning usually involves a reining-in of marketing costs. But Sucampo CEO Peter Greenleaf is doing the exact opposite. In the firm’s second-quarter earnings call, he announced the company is “doubling-down” on Amitiza, the constipation treatment which has been on the market for eight years and which will soon face the threat of generic competition.
Amitiza owns just 1% of the overall constipation market, which includes a number of OTC options, a representative for the drugmaker told MM&M. Sucampo co-markets Amitiza with Takeda.
back to top 

Medical equipment supplier Becton Dickinson & Co (BDX.N) has agreed to buy CareFusion Corp (CFN.N), a maker of infusion pumps and other medical devices, for $12.2 billion in cash and stock, marking the latest multibillion-dollar healthcare sector deal.
Becton said on Sunday it would pay a total of $58.00 a share – $49.00 in cash and 0.0777 of a share of Becton Dickinson – for each share of CareFusion, representing a premium of 26 percent to the closing price on Oct. 3.
back to top 

Pipeline updates are highly awaited events in the pharma/biotech sector as they play an important role in deciding whether or not to invest in a particular company. These updates provide information on experimental drugs and at times give an insight into the commercial potential of the candidate once it is successfully developed and commercialized.
Roche (RHHBY – Analyst Report) specializes in cancer drugs. Immunotherapy has received a lot of attention in recent times as pharmaceuticals majors focus their research and development efforts on the same.
back to top 

GlaxoSmithKline has just launched a $5 million Innovation Challenge Fund to advance open-access technology in the bioelectronics space.
The funding’s aimed toward biz-savvy academics and startups that are working to create a new class of treatments that aren’t necessarily pills or injections, but rather are mini implantable devices. GSK says:”The hope is that these devices could be programmed to read and correct the electrical signals that pass along the nerves of the body, to treat disorders as diverse as inflammatory bowel disease, arthritis, asthma, hypertension and diabetes.”
back to top 

University of Maryland, Baltimore County, and University of Maryland, College Park will work with MITRE Corp. on a new cyber security research and development center in Montgomery County.
McLean, Va.-based MITRE was selected by the National Institute of Standards and Technology for a $29 million award to operate a new federally funded research and development center dedicated to cyber security. The center will be housed at the National Cybersecurity Center of Excellence in Montgomery County.
back to top 

Tasly Pharmaceuticals, Inc. made its official debut at the Natural Products Expo East 2014, held from September 17-20, 2014 in Baltimore, MD. As the leading East Cost trade show in the natural, healthy and organic products industry, attracting 22,000 professionals and representing 33% of the natural products industry, Expo East 2014 was a perfect opportunity for Tasly to introduce Deepure, its inaugural line of nutraceuticals. The line includes three condition-specific, whole-food and herb-based formulas, namely, ProHeart PLUS, ImmunoPower PLUS, and Re-Memory PLUS. All Deepure nutraceuticals are gluten-free and made without chemicals, preservatives, artificial colors, flavors, sweeteners, or gelatin. Deepure will be available in stores nationwide later this Fall.
Tasly also participated in two very attractive marketing and sponsorship opportunities located in New Products Pavilion, including Best of East Press Showcase and New Products Showcase.
back to top 

To attend a NICE/UKTI Scientific Advice Seminar for developers of devices and diagnostics.
Learn how to prepare for an evaluation by NICE, the UK’s health technology assessment body.
The seminar will cover:
- “Value” from the perspective of NICE
- How value propositions link to the need for specific evidence
- The types of evidence considered by NICE
- The general principles of health technology assessment (HTA)
- Advantages of doing business in the UK
Who should attend?
- Developers of medical technologies, in particular SMEs
- Investors in the field of medical technologies
- Anyone new to the world of health technology assessment
- Academics, scientists, or clinicians who want to know more about how NICE assesses the value of innovative products
The seminar program is funded by UKTI to allow you free event registration (standard event rate – $550)
Read More
|
|
|
 ROCKVILLE AND BALTIMORE, MARYLAND, September 30, 2014 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today that venture capitalist, Tania Fernandez, Ph.D., has joined the BHI team as a strategic advisor. Dr. Fernandez will be a member of the management team for a new BioHealth Gap Fund, which will provide up to $50 million in seed and early-stage equity investments to therapeutics, medical device, diagnostics, and health IT companies in Maryland. Additional BioHealth Gap Fund management team members include Richard Bendis, Ram Aiyar, Todd Chappell, and Ken Malone, who each bring domain knowledge and industry access to the fund. ROCKVILLE AND BALTIMORE, MARYLAND, September 30, 2014 – BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today that venture capitalist, Tania Fernandez, Ph.D., has joined the BHI team as a strategic advisor. Dr. Fernandez will be a member of the management team for a new BioHealth Gap Fund, which will provide up to $50 million in seed and early-stage equity investments to therapeutics, medical device, diagnostics, and health IT companies in Maryland. Additional BioHealth Gap Fund management team members include Richard Bendis, Ram Aiyar, Todd Chappell, and Ken Malone, who each bring domain knowledge and industry access to the fund.
“Dr. Fernandez has ten years of experience as a venture capitalist in the life sciences/biotechnology industry. Her work in Silicon Valley, along with her research experience at the National Cancer Institute, makes her a tremendous asset to the BHI team,” said Richard Bendis, BHI President & CEO. “Dr. Fernandez brings a West Coast investment perspective, and she will have an active role in helping to manage the BioHealth Gap Fund. She will also support our BHI Entrepreneurs-in-Residence and clients: helping our startups to grow and raise strategic funding.”
Dr. Fernandez previously was a venture capitalist with Burrill & Company, a $1.5 billion fund with a portfolio of 103 companies in the biotechnology/life science industry. Dr. Fernandez has over sixteen years of industry experience with the ability to successfully identify and assess promising scientific technologies/products and business models for venture investments across the sectors of therapeutics, diagnostics, next-generation sequencing and healthcare delivery. She has led and managed investments through both operational and Board positions. Roche acquired her lead investment, Bioimagene, for $100 million within two years of investment. Dr. Fernandez plays an active role in training and mentoring entrepreneurs in Silicon Valley and provides strategic advisory services to companies that range from startups to revenue-driven companies.
BHI’s BioHealth Gap Fund is anticipated to be a $50 million fund that will invest in disruptive companies throughout Central Maryland looking to close the gap between seed and early-stage financing. The fund will focus on therapeutics, medical devices, diagnostics, and health IT companies, and provide seed and early-stage equity investments along with follow-on capital for growth to help companies exit successfully.
“It is an honor to have the opportunity to work with BHI and to help manage the new BioHealth Gap Fund,” said Dr. Fernandez. “The potential to serve a pressing need in the current life sciences funding landscape in Maryland is tremendous, and I am delighted to be a part of it.”
About BioHealth Innovation, Inc.
BioHealth Innovation, Inc., is a regional innovation intermediary focused on commercializing market-relevant bio-health innovations and increasing access to early-stage funding in Maryland.
back to top 
 On September 10, 2014, Tasly Pharmaceuticals, Inc. (Tasly) and BioHealth Innovation, Inc. (BHI) signed a Memorandum of Agreement (MOA) which aims to advance their business partnership. The MOA also lists specific near- and long-term collaboration activities between the two parties. On September 10, 2014, Tasly Pharmaceuticals, Inc. (Tasly) and BioHealth Innovation, Inc. (BHI) signed a Memorandum of Agreement (MOA) which aims to advance their business partnership. The MOA also lists specific near- and long-term collaboration activities between the two parties.
back to top 

U.S. Senators Ben Cardin and Barbara A. Mikulski (both D-Md.) today announced that the Department of Labor (DOL) has awarded $14,957,899 in federal funding to fourteen Maryland community colleges as part of the Trade Adjustment Assistance Community College and Career Training (TAACCCT) initiative. The TAACCCT program allows community colleges and other institutions to expand their ability to provide quality education and job training programs in two years or less.
Of the nearly $15 million, Montgomery College received $5,371,743 to lead and fund the Cyber- Technology Pathways Across Maryland (CPAM) Consortium. CPAM is comprised of fourteen Maryland community colleges. It seeks to train and educate Trade Adjustment Assistance workers, veterans, the un- and –under employed and low skilled adults. The Consortium will work to connect participants with employers looking to fill thousands of unfilled job openings. CPAM focuses on bringing women and other underrepresented populations into the growing fields of cyber technology and cyber security.
back to top 
 Rich Bendis was interviewed on 99.1 WNEW Newstime about partnering with Roche and describing what it is that BioHealth Innovation does for Maryland. Rich Bendis was interviewed on 99.1 WNEW Newstime about partnering with Roche and describing what it is that BioHealth Innovation does for Maryland.
back to top 
 This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation. This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.
back to top 
 Tuesday, October 07, 2014, 08:00am – 10:00am Tuesday, October 07, 2014, 08:00am – 10:00am
At the forefront of innovation, the Consumer Electronics Association (CEA) unites 2,000 companies in the consumer technology industry and owns and produces the world’s largest annual innovation tradeshow, the International CES®. Gary Shapiro, the president and CEO of CEA, knows innovation and is the go-to source to tell you what’s cool in consumer electronics. Ask him what’s in this year and he’ll talk about Ultra HD 4K TV, 3-D robotics, and wireless health. He’ll also mention that the biggest drivers of consumer electronics are products that didn’t even exist a few years ago. Join Gary as he takes you on a spirited discussion about the importance of innovation in the U.S. economy, green technology, and keeping the American dream alive.
Get a look inside Shapiro’s passion for innovation and see what it’s like to be at the forefront of a multi-billion dollar industry.
back to top 

Tuesday, October 7, 2014 from 6:00 PM to 8:00 PM (EDT)
Rockville, Maryland
In February 2013, The Sunshine Act was included as the Transparency Reports and Reporting of Physician Ownership or Investment Interests section of the Patient Protection and Affordable Care Act (ACA). The Sunshine Act requires manufacturers of drugs, medical devices, and biologicals that participate in U.S. federal healthcare programs to report certain payments and items of value (typically $10 or more and totaling $100 annually or greater) given to physicians and teaching hospitals. Failure to stay in compliance may result in fines ranging from $10,000 to $1,000,000 annually. Whether you are a practicing physician or your startup has a medical device, drug, or related product, you are impacted by the ACA’s Physician Payments Sunshine Act. In July, CMS proposed removing the reporting exemption for any payments or transfers of value made to physicians who participate in accredited CME programs. We’ll talk with experts in compliance and policy who will share their experiences in implementing these new policies and how you can understand the implications of the law, dispute inaccuracies, and stay in compliance!
back to top 

Thursday October 9, 2014 9:00 AM – 3:15 PM
The DC I-Corps Fall 2014 Regional Cohort officially kicks off on October 9th at the Microsoft building in Chevy Chase, MD. Please register to join us for our Showcase lunch, in which successful teams from previous cohorts will present their businesses and discuss lessons learned from the I-Corps program.
Showcase agenda:
- Welcome and lunch
- Introduction of incoming Fall cohort teams
- Presentations by Accelerator teams
- Q&A
About DC I-Corps: DC I-Corps is a regional program designed to foster, grow and nurture an innovation ecosystem in the nation’s capital, the nearby states of Maryland and Virginia, and the mid-Atlantic region. The program is sponsored by the National Science Foundation (NSF) and jointly run by the University of Maryland College Park, George Washington University, Virginia Tech and Johns Hopkins University. The program provides real world, hands-on training on how to successfully incorporate innovations into successful products. The ultimate goal is to create a new venture or licensing opportunity for program participants.
back to top 

The National Institutes of Health and the U.S. Food and Drug Administration will receive a top national award for the year’s most outstanding intellectual property licensing deal, for technology transfer of a pioneering, low-cost meningitis vaccine launched in sub-Saharan Africa. The 2014 Deals of Distinction Award will be presented to the two federal agencies and their collaborators by the Licensing Executives Society at the society’s 50th annual meeting, Oct. 5-8 in San Francisco.
back to top 
Johns Hopkins University named Leslie Ford Weber director of campus, government and community affairs for Montgomery County in Rockville.
She succeeds Elaine Amir, who retired in September 2013.
Weber had been interim executive director of the campus since October and director of government and community affairs for Suburban Hospital in Bethesda since July 2011.
back to top 

Wednesday, October 1st, is the deadline to apply for the next round of BioMaryland Center biotechnology development awards. The BioMaryland Center is partnering this year with Maryland’s Dept. of Health and Mental Hygiene (DHMH) and the Center for Medical Technology Policy (CMTP) to incorporate improved health care quality and cost reduction criteria in the selection process for the Center’s annual Awards program.
A total of $1M will be awarded on a competitive basis to projects, $50,000-200,000 each, advancing technologies toward commercialization–with preference given to projects which improve patient outcomes and reduce costs.
BioMaryland, DHMH and CMTP will provide ongoing advice and support to the teams whose projects are selected for funding to address technical, scientific, regulatory and reimbursement issues that may be encountered during the development process.
back to top 

Nationally, young firms play a central role in the creation of new employment opportunities. High-tech companies are particularly important to job creation: over 9% of average annual net job creation from 1990-2011 is due to high-tech firms younger than 5 years old. All private firms younger than 5 years old created less than 6% of average annual net job creation.
Young, innovative companies have generated the majority of new jobs in Greater Baltimore over the past 5 years. This trend is consistent with similar metropolitan regions and the coun- try as a whole. New technologies, research, and ideas spawn new teams, divisions, and entire companies. Continuing to support and encour- age innovation is imperative to sustaining growth in the Greater Baltimore economy.
back to top 

Circulomics Inc has been awarded a Phase I Small Business Innovative Research (SBIR) grant by the National Institutes of Health (NIH) to develop its Nanobind DNA and RNA isolation technology for formalin-fixed, paraffin-embedded (FFPE) samples. This grant was made by the National Institute of Environmental Health Sciences to create a novel method for extracting molecular information from archived tissues. FFPE sample archives contain a wealth of molecular biomarker information that can be compared to standard histological analysis and correlated to clinical outcomes. However, the DNA and RNA isolated from FFPE samples are often degraded due to damage from the FFPE preservation process as well as contaminated by residual formalin and paraffin wax.
back to top 

Emergent BioSolutions Inc. (NYSE:EBS) today announced the initiation of the pivotal non-clinical efficacy study to demonstrate that BioThrax® (Anthrax Vaccine Adsorbed) manufactured at large scale in the company’s new modern facility, Building 55, is comparable to the BioThrax currently manufactured in its approved facility, Building 12. Data from this study will be used to support licensure of Building 55. BioThrax is the only vaccine licensed by the U.S. Food and Drug Administration (FDA) for the prevention of anthrax disease.
back to top 

ATCC increases focus on scientific reproducibility and leverages technological advances with new senior executive appointments
ATCC, the premier global biological materials resource and standards organization, is pleased to announce two important appointments to the senior leadership team. Barbie Bigelow has joined ATCC as Executive Vice President of Strategy and Technology and Dr. Maryellen de Mars also joins as the Senior Director for the Standards Resource Center. Both roles are new positions at ATCC.
back to top 

AstraZeneca announced today that its global biologics research and development arm, MedImmune, has received fast track designation from the US Food and Drug Administration (FDA) for its investigational monoclonal antibody (mAb) MEDI3902 for the prevention of nosocomial pneumonia caused by Pseudomonas aeruginosa (P. aeruginosa).
Pseudomonas aeruginosa (P. aeruginosa) causes serious disease in hospitalised patients. The FDA’s Fast Track programme is a process designed to expedite the development and review of drugs to treat serious conditions and fill an unmet medical need.
back to top 

There are two prevailing perceptions about innovation and start-ups: first, they are all tech driven, and second, they originate from just a few regions — chief among them, Silicon Valley. I’ve seen firsthand that innovation can happen anywhere, and that it is accelerating in places that typically don’t grab headlines. And I have met hundreds of entrepreneurs living in cities in “flyover country” that are building great companies and creating jobs in a wide range of industries.
back to top 

A drug used to treat advanced breast cancer has had what appears to be unprecedented success in prolonging lives in a clinical trial, researchers reported on Sunday.
Patients who received the drug — Perjeta, from the Swiss drug maker Roche — had a median survival time nearly 16 months longer than those in the control group.
back to top 

Medimmune, the global biologics research and development arm of Anglo-Swedish drug major AstraZeneca (LSE: AZN), has entered into a collaboration to establish a joint lab in Cambridge, UK, with Cancer Research Technology, the commercial arm of Cancer Research UK.
The new laboratory will be the first partnership of its kind of both organizations, and will focus on the discovery and development of biologic cancer treatments over an initial five-year period.
back to top 

Officials from local and state government as well as business and education attended a virtual ribbon cutting on Wednesday to celebrate the opening of the new $71 million Center for Communications and Information Technology at Maryland’s Frostburg State University.
“This is one of the most technologically advanced learning centers in the United States. Every inch of this building fosters learning,” said Jonathan Gibralter, FSU president.
back to top 

The Baltimore Business Journal has selected honorees for its first-ever Health Care Innovators awards.
These winners created new health care products and strategies that have made health care more accessible, efficient and effective. They will be featured in the Nov. 7 issue of the Baltimore Business Journal and will be recognized at a breakfast at the Hotel at Arundel Preserve on Nov. 7. Farzad Mostashari, the former national coordinator for health IT for the federal health department, will be the keynote speaker at the event.
back to top 

The FLC is pleased to offer its latest on-demand, and FREE, e-learning course, “Introduction to CRADAs”! If you’re new to tech transfer and need to know more about one of its most important mechanisms, this introductory-level e-course is perfect for you.
Available free of charge and at your convenience, the course covers essential CRADA knowledge:
- CRADA function and purpose
- What CRADAs can accomplish
- When CRADAs are used.
back to top 

GSK and venture capitalists Avalon have launched two early-stage R&D biotechs in San Diego, California.
Silarus Therapeutics and Thyritope Biosciences will each receive $10m (€8m) in a Series A financing round to investigate the hormone behind anaemia, and anautoimmune disease.
back to top 

With so much venture capital being foisted onto the digital health space, it’s beginning to beg the question: how long will this last, can it sustain itself, and what’s an entrepreneur to do? And, what are the implications for emerging companies versus traditional healthcare companies and systems?
Those were just a few of the burning questions discussed at Health 2.0‘s Pre, Post, M&A IPO panel held in Santa Clara.
back to top 

American University’s Kogod School of Business on Friday plans to show off its new on-campus start-up incubator, the latest in a string of co-working spaces to pop up in and around the nation’s capital.
The incubator, one of the key components of the school’s recently announced Sustainable Entrepreneurship and Innovation Initiative, aims to provide current students and recent graduates with work space and pair them with a business mentor to help get their fledgling ventures off the ground. In addition, each business team will received a $1,500 grant to cover some initial start-up costs, such as the legal work necessary to incorporate a company.
back to top 
New Enterprise Associates, a venture capital firm that has backed the likes of Groupon and Salesforce, is now investing in a biotechnology company developing a treatment for cancer.
The venture firm, which has a significant health care business, has led a $104 million financing round in Adaptimmune Limited, the company announced on Wednesday evening. The round, at the Series A stage, is believed to be among the largest for a biotechnology company at this early point of its development.
back to top 

The Atlantic Vaccines and Immunotherapeutics Summit is a first-of-its-kind event to showcase Maryland’s global leadership as an epicenter of vaccine innovation, development and commercialization. The Tech Council of Maryland is presenting this conference to bring together the industry’s foremost researchers and business leaders for the purpose of educating, sharing, and collaborating on important issues affecting the new generation of vaccines.
The Summit’s agenda will focus on a spectrum of topics including global R&D; government priorities and challenges; regulatory processes and policies; university and academic development; models for regional synergy; and vaccine market impact. We invite you to join us for this two-day event on May 7-8, 2015 at the Bethesda North Marriott in Bethesda, Maryland.
back to top 

Winners of the second annual Baltimore Innovation Awards included a civic hacker and a youth outreach organization.
The awards, a centerpiece of Baltimore Innovation Week’s Innovation Celebration, held Friday outside Under Armour headquarters in Tide Point, were announced in a brief presentation by Christopher Wink, editorial director and cofounder of Technical.l
back to top 

The success of Alibaba’s blockbuster initial public offering in the U.S. has catapulted founder Jack Ma into the limelight and given him the status of China’s richest man.
The making of Alibaba began in 1999 when e-commerce was unheard of in China. Recalling the days when he started the venture with 17 friends in his flat in the South-eastern Chinese city of Hangzhou, Ma once said: “I called myself a blind man riding on the back of blind tigers.” 15 years later, the firm is the dominant player in China’s e-commerce space. Between its two main online marketplaces Taobao and TMall, Alibaba accounts for nearly 80 percent of the mainland’s e-retail transactions.
back to top 

Tanisha Robinson is on tech startup No. 4 or so, with two running simultaneously at the moment, and aiming to make the latest a billion-dollar business. Here’s her best test to find if someone has what it takes to become an entrepreneur:
“If I were to take your wallet and your phone and your keys, and say you have to survive on your wits for a week,” she said.
back to top 

Being an entrepreneur can mean a demanding, unpredictable schedule; spreading oneself way too thin; and trying to pull off tremendous, seemingly impossible feats. This sometimes leads to burnout, and even if we don’t want to admit it, unhappiness. Matthew Toren penned a piece for Entrepreneur about habits of healthy, happy, and wise entrepreneurs. One of the best practices that leads to happiness? Setting and enforcing boundaries. Sounds obvious, but definitely easier said than done when you’re trying to please everyone from employees to spouses. Toren recommends:
back to top 

In the 24 years since the founding of the Georgia Research Alliance, federally-funded research and development grants to Georgia’s universities has increased five-fold.
The state’s total share of federal research funding increased to nearly 3 percent, ranking 12th and one of only five of the top 16 states that is increasing its market share.
back to top 

The Texas Medical Center has more square footage, more doctors and more hospitals than any other medical center in the country. However, that has not translated into product commercialization necessarily.
Its new accelerator may be the catalyst that changes that, experts say.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 7
Bethesda Country Club

October 7
Johns Hopkins University Montgomery County Campus

October 9

October 9
Microsoft Corporation

October 15
Johns Hopkins University – Montgomery County
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

 Tasly Pharmaceuticals, Inc., a global leader in pharmaceuticals, biologics, and nutraceuticals, held a ribbon-cutting ceremony on September 3, 2014, at Tasly’s corporate offices in Rockville, MD. Tasly Pharmaceuticals, Inc., a global leader in pharmaceuticals, biologics, and nutraceuticals, held a ribbon-cutting ceremony on September 3, 2014, at Tasly’s corporate offices in Rockville, MD.
The ceremony marked the official launch of Tasly’s Rockville location as the North American headquarters of Tasly Holding Group, a twenty year old global technology company established in China.
More than 100 distinguished guests and dignitaries were in attendance, including the Chairman and the President of Tasly Holding Group, China’s Embassy Counselor, Maryland state officials, Maryland biotechnology and pharmaceutical executives, and high-level regional media representatives, among others.
back to top 

This editor attended TEDMED 2014 in DC and had a chance to speak with some of the innovative start ups in the Hive, a showcase of new medical technologies.
BeneVir has developed an impressive immunotherapy for cancer that has the potential to become a lasting, durable cure to the disease. While other existing immunotherapy treatments exist, these solutions only help the immune system scour the body for known cancer cells afflicting a patient. These solutions fail to educate the immune system on how to predict, recognize, and kill cancer mutations that avoid the immune system and lead to a recurrence of the disease. BeneVir’s solution uses a natural human virus whose genes have been altered to allow the virus to target both the original cancer cells and their mutations. Prior to becoming a company, BeneVir’s founders originally developed their immunotherapy concept and licensed the solution to T-Vec, which was acquired by Amgen for $1B in 2011. This original concept recently completed a Phase 3 clinical trial showing a 16% durable cure for melanoma. With this success, BeneVir was formed to expand on the original innovation with what the founder calls version 2.0 plus. With deep technical knowledge, BeneVir is refining their original immunotherapy solution to become an even more potent cancer therapy.
back to top 

OpGen, Inc., a whole-genome analysis company developing and commercializing a complete suite of break-through products and services based on its proprietary Whole Genome Mapping technology, announced today the appointment of Vadim Sapiro as chief information officer. In addition, the company announced the promotion and appointments of several key members of the executive committee. The organizational changes have been made to strengthen the management team and accelerate OpGen’s development and commercialization strategy. The company’s products enable rapid, accurate, high resolution whole genome analysis of microbes as well as more cost effective and accurate sequence assembly and finishing of human, animal, plant and microbial genomes.
back to top 
 The 2014 NIH Research Festival, the annual showcase of the NIH Intramural Research Program, will be held Sept. 22-24. This year’s theme is “The Era of the Brain.” The festival kicks off with an opening plenary session at 10 a.m. on Monday, Sept. 22 in Masur Auditorium, Bldg. 10. The plenary session will include remarks by NIH director Dr. Francis Collins, followed by the FARE awards ceremony and scientific talks by Drs. Antonello Bonci (NIDA) and Mark Hallett (NINDS). The 2014 NIH Research Festival, the annual showcase of the NIH Intramural Research Program, will be held Sept. 22-24. This year’s theme is “The Era of the Brain.” The festival kicks off with an opening plenary session at 10 a.m. on Monday, Sept. 22 in Masur Auditorium, Bldg. 10. The plenary session will include remarks by NIH director Dr. Francis Collins, followed by the FARE awards ceremony and scientific talks by Drs. Antonello Bonci (NIDA) and Mark Hallett (NINDS).
back to top 

Oculus CEO Brendan Iribe first began working with computers in the 1980s, just as personal computers were entering homes. “I always said that it was the most exciting, amazing time,” he says. “We had no idea that in some number of years, a personal computer would within two physical taps, revolutionize, say, the entire taxi service industry.”
He has since revised his definition of the most exciting time to be in computer science to “now.”
back to top 
 Wednesday, October 15, 2014, 08:30am – 11:45am Wednesday, October 15, 2014, 08:30am – 11:45am
Johns Hopkins University – Montgomery County
This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.
Contact Ethan Byler at ebyler@biohealthinnovation.org if you are interested in scheduling one-on-one meetings.
More Information
back to top 

The 2014 Innovation2Commercialization Conference will be held on October 23rd at the Universities at Shady Grove in Rockville. This is our third year and we would like to ask you participate again to support and encourage the innovators, scientists, technologists and entrepreneurs in our area.
The conference will be held from 7:30am-3:30pm, with three panels: Innovation, Commercialization and Financing, and a special Keynote Speaker – Rachel King, CEO, GlycoMimetics. The program will spotlight serial entrepreneurs and successful tech transfer to product from the Naval Research Laboratory and The Johns Hopkins University – including a full panel discussion on the successful development to exit for Amplimmune.
As Partners in the past, we hope you found participation in the I2C Conference productive and plan to join us again this year.
back to top 

AstraZeneca and Eli Lilly and Company have reached an agreement for the development and commercialization of the oral beta secretes cleaving enzyme (BACE) inhibitor, AZD3293, which might become a viable treatment for Alzheimer’s disease. The oral therapy is expected to prevent the formation of amyloid plaque, which is comprised of peptides called amyloid beta, and slow the disease progression.
The agreement established stipulates that AstraZeneca will pay up to $500 million in development and regulatory milestone payments. Lilly announced that it is planning on receiving the first milestone payment of $50 million in the first half of 2015. Future costs will be equally shared by both companies, including net global revenues post-launch.
back to top 

Tuesday, October 7, 2014 from 6:00 PM to 8:00 PM (EDT)
Rockville, Maryland
In February 2013, The Sunshine Act was included as the Transparency Reports and Reporting of Physician Ownership or Investment Interests section of the Patient Protection and Affordable Care Act (ACA). The Sunshine Act requires manufacturers of drugs, medical devices, and biologicals that participate in U.S. federal healthcare programs to report certain payments and items of value (typically $10 or more and totaling $100 annually or greater) given to physicians and teaching hospitals. Failure to stay in compliance may result in fines ranging from $10,000 to $1,000,000 annually. Whether you are a practicing physician or your startup has a medical device, drug, or related product, you are impacted by the ACA’s Physician Payments Sunshine Act. In July, CMS proposed removing the reporting exemption for any payments or transfers of value made to physicians who participate in accredited CME programs. We’ll talk with experts in compliance and policy who will share their experiences in implementing these new policies and how you can understand the implications of the law, dispute inaccuracies, and stay in compliance!
back to top 

The Johns Hopkins University in Baltimore and other research institutions closer to Greater Washington have received hundreds of thousands of dollars from the ALS Association in the past few years.
Early indications are that some are likely to see even more in the future as contributions to the viral “Ice Bucket Challenge” have surpassed $113 million.
back to top 

Accepting Applications for InvestMaryland Challenge!
Could $100,000 help jumpstart your startup? Well, Maryland’s national business competition is back for the third year!
Since 2013, InvestMaryland Challenge has awarded nearly $1.5M in prizes to innovative startup companies. Prizes have included cash grants, incubator space, marketing and legal services, and opportunities to pitch investors.
Start an application today and your business could be on its way to accepting the $100,000 Grand Prize!
back to top 

An entrepreneurial and innovative spirit filled the conference room Tuesday, Sept. 16, at the Talbot County Free Library in Easton.
As part of Innovation Week, the third annual Startup Maryland Pitch Bus tour made a stop at the library. Business representatives from the additive manufacturing industry, commonly known as 3-D printing, also had 3-D printers and products on display.
The pitch bus offered an opportunity for area entrepreneurs to make a video for public viewing on YouTube to compete for venture investment.
back to top 

A competition by Washington, D.C.-based incubator 1776 in a global search for the finest startup has set down the groundwork for its annual Challenge Cup in search of technology and healthcare startups. It is working with incubators in 16 cities around the world to identify early-stage companies across education, energy health and environment in a new twist to the competition called ChallengeX.
It begins in Washington, D.C. on October 21 and in Chicago on October 28, according to a company statement. In addition to those cities, it is working with incubators in Sydney, New York City, Tel Aviv, Amman, Santiago, Nairobi, Mumbai, Austin, Toronto, Boston, Berlin, Dublin, San Francisco and China. Each city will have four winners in each of the categories and advance to a week long festival in May when entrepreneurs for these companies have a chance to pitch investors, receive mentoring and meet policymakers. The intention of the Challenge Cup is to search for meaningful technology that’s also scalable.
back to top 

More than 300 entrepreneurs are expected to vie for between 150 and 175 spots on the Startup Maryland bus to film a pitch video of their idea.
Now in its third year, the Startup Maryland’s Pitch Across Maryland tour will make 27 stops where entrepreneurs can film a short pitch video about their business. A panel of judges selects eight finalists, of which a winner and runner up are entered into a finalist round of the state’s InvestMaryland Challenge business competition.
back to top 

DC I-Corps, a new, NSF-supported program designed to foster, grow and nurture an innovation ecosystem in the Mid-Atlantic Region, is now accepting applications for its fall cohort, beginning on October 9. Applications will be accepted on a rolling basis up until that date. Apply here.
Open to research teams and technology entrepreneurs from universities, federal laboratories, agencies and the general community, the free program guides researchers in exploring the commercial potential of their inventions.
back to top 
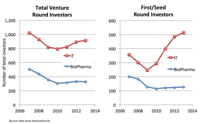
The ever-shrinking number of biotech venture capital firms was a common refrain during the 2008-2012 period; it’s true that a large number of firms went under, closed their doors for new investments, or moved into zombie status. I wrote on the subject back in July 2012 (here). The biotech investing environment then, with a relatively bleak IPO landscape and venture funding off some 40% from the prior year, was very different from the past two years with an “open” IPO window and steady pace of venture funding.
back to top 

On September 16, the American Academy of Arts and Sciences released a report entitled Restoring the Foundation: The Vital Role of Research in Preserving the American Dream. The report addresses the link between scientific research and advancing innovation and prosperity, and contains recommendations for strengthening its role to address challenges in the 21st Century. The recommendations fall under three overarching objectives: securing America’s leadership in science and engineering research; ensuring that the American people receive the maximum benefit from federal investments in research; and regaining America’s standing as an innovation leader by establishing a more robust national government-university-industry research partnership.
back to top 

As of 2014, Deloitte is the largest professional services firm in the world by revenue. What began in the 19th century as stand-alone accounting offices has merged and evolved into a partnership that encompasses 200,000 employees in more than 150 countries around the globe.
But size alone doesn’t make a company great, of course. Things like satisfying clients, increasing opportunities for personnel, and performing a vital function for society make a company great. When employees, customers, investors, and the community benefit from a company’s services, that’s what makes it stand out from the rest.
back to top 

Washington state’s Providence Health & Services today said it is launching a $150 million venture capital fund to help spur innovation across the healthcare system that lowers costs and leads to better outcomes.
Coincidentally, it’s the second VC fund announced in as many days on the West Coast, following yesterday’s announcement from the University of California.
back to top 

FasterCures, part of the Milken Institute dedicated to improving drug development by encouraging greater patient involvement is searching for digital health entrepreneurs to pitch at its upcoming conference, Partnering for Cures. The group is looking for collaboration opportunities between biotech and digital health, partly to find more effective ways to improve clinical trial recruitment.
Entrepreneurs for Cures will spotlight entrepreneurs who are developing digital and mobile health technology that boost patient participation in biomedical research and development and those looking for partnerships with patient communities to demonstrate their product’s capabilities. It’s particularly interested in companies pursuing solutions geared to chronic conditions, acute and infectious diseases, mental illness, genetic conditions.
back to top 

Since its passage more than two years ago, the Jumpstart Our Business Startups (JOBS) Act, which was designed to decrease regulatory burdens for raising capital, has spurred more than 110 IPOs among biotechnology companies alone. These are companies working to find cures for cancer, cardiovascular disease, blood disorders, and infectious diseases, among other conditions. The Securities and Exchange Commission (SEC) is now in the process of finalizing another important provision of the law that could further encourage biotech capital formation and investment.
back to top 

Dutch venture capitalist Aglaia has announced the launch of Aglaia Oncology Fund II. Investors contribute a total of $65 million to the fund, whose target size is $80 to $100 million. Like its predecessor Aglaia Oncology Fund I, the fund is backed by high-net-worth families. The group of investors has now been expanded to include several institutional investors, including the European Investment Fund (EIF), which has made a substantial commitment. Aglaia is also one of the funds selected under the Dutch Venture Initiative, an investment fund recently launched by the Netherlands Ministry of Economic Affairs.
Through the new fund, Aglaia will be investing in an estimated ten to fifteen biotechnology start-ups which are in the process of developing groundbreaking technologies aimed at preventing and curing cancer.
back to top 

The intersection of health and big data is getting more crowded. San Mateo, Calif.-based Lumiata is the latest company to get venture backing for technology that crunches data with the aim of improving medical care, Yuliya Chernova reports for Dow Jones VentureWire.
The company has already run an analysis of some 100,000 BlueCross BlueShield patients that found about 10,000 people who were very likely to have congestive heart failure, even though they were undiagnosed. Now the insurance provider, which is also an investor in the company, is alerting doctors who have those patients about this potential missed diagnosis.
back to top 

AS a former physician, I shivered a bit when I heard Dr. Vivek Wadhwa say he would rather have an artificial-intelligence doctor than a human one. “I would trust an A.I. over a doctor any day,” he proclaimed at a recent health innovation conference in San Francisco, noting that artificial intelligence provided “perfect knowledge.” When asked to vote, probably a third of those in attendance agreed.
But it made sense: Dr. Wadhwa is a professor, entrepreneur and technology visionary. What’s more, the conference took place in San Francisco, where faith in the power of technology and data to solve problems holds unshakable sway.
back to top 

Recognizing the continuing evolution of New Jersey’s life sciences industry, BioNJ today released a comprehensive report that documents the ongoing growth of the biotechnology sector in the State and combines and assesses the contributions of the entirety of the life sciences sector to New Jersey’s economy, including employment and economic impact.
BioNJ conducted the study in partnership with EY (formerly known as Ernst & Young) the Edward J. Bloustein School of Planning and Public Policy (Bloustein School) and the New Jersey Department of Labor and Workforce Development’s (LWD) Office of Research and Information. To access the study go to: http://www.bionj.org/wp- content/uploads/2014/09/Industry-Study-9-19-14-Final-Final.pdf.
back to top 
Earlier this year, both Apple and Google presented competing visions for how we’ll use apps and wearables to gather data about our bodies and share it via our phones. Apple called its software HealthKit, while Google presented Google Fit.
If things had gone according to plan on Wednesday, Apple would be enjoying a head start over Google, with HealthKit released along with iOS 8, its new mobile software for iPhones and iPads. (Google Fit is still unreleased beyond a “developer preview.”)
back to top 

Some of the trends in American health care are obvious: Managing costs in the age of Obamacare, patients using online information to take charge of their own health and wellness, and finding ways to deliver care in settings other than hospitals and clinics.
Those trends become specific, sometimes knotty, challenges for people engaged in building the tools needed to effectively, efficiently and safely deliver health care.
Envisioning, designing and manufacturing medical devices – which range from robots to sensors, and from surgical instruments to software that allows devices to communicate with one another – was the topic of a conference Thursday and Friday in Chicago.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 22-24
NIH Building 10

September 25
MedImmune

October 7
Bethesda Country Club

October 7
Johns Hopkins University Montgomery County Campus

October 9
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI) announced today that Roche Pharma Research and Early Development (pRED) and BHI have entered into an agreement to advance healthcare technologies coming from academic institutions, federal laboratories and startups based in Central Maryland. Under the terms of the agreement, BHI will identify health technologies that Roche will evaluate for potential research, development and commercialization opportunities. Priority areas of interest will include oncology, neuroscience, ophthalmology, rare diseases, immunological and infectious diseases. Financial terms for the agreement were not disclosed.
The agreement was initiated through Roche pRED Academic Relations and Collaboration (ARC) group, which is led globally by Juan Carlos Lopez. “Sourcing of technologies from academia, federal labs and early-stage startups can be a challenging and lengthy process,” said Lopez. “As we seek to expand our capabilities to source and screen technologies aligned with our strategic interests, BHI is an ideal partner to support identification of external opportunities in a broad, systematic fashion.”
back to top 
 Tasly Pharmaceuticals, Inc (Tasly) and BioHealth Innovation (BHI) co-organized the first Tasly Meeting of CEOs on Tuesday, September 2, 2014 at the Tasly facility in Rockville. Attendees included more than 25 CEOs and company representatives from local Maryland-based BioTech and BioPharma companies. This was the first public event conducted through Tasly and BHI’s newly initiated partnership. Tasly Pharmaceuticals, Inc (Tasly) and BioHealth Innovation (BHI) co-organized the first Tasly Meeting of CEOs on Tuesday, September 2, 2014 at the Tasly facility in Rockville. Attendees included more than 25 CEOs and company representatives from local Maryland-based BioTech and BioPharma companies. This was the first public event conducted through Tasly and BHI’s newly initiated partnership.
Tasly is an innovation-based, high-tech healthcare and pharmaceutical enterprise and world-leader in the research and product development (R&D) of Chinese herbal medicine – the leader in the natural way to modernize medicine, with a North American headquarters in Rockville, Maryland, . Tasly and BHI have a formalized partnership which aims to co- organize and coordinate joint BioHealth associated events to promote professional networking, business growth, and technology and product commercialization.
back to top 
 Wednesday, October 15, 2014, 08:30am – 11:45am Wednesday, October 15, 2014, 08:30am – 11:45am
Johns Hopkins University – Montgomery County
This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.
Contact Ethan Byler at ebyler@biohealthinnovation.org if you are interested in scheduling one-on-one meetings.
More Information
back to top 

Sebastian Seiguer had already founded emocha Mobile Health by the time his company began its four-month term in January 2014 as part of DreamIt Health Baltimore, an accelerator for developing health IT and health care startups that graduated its first class in May. But Seiguer’s venture, an online remote patient management platform for clinicians to monitor patients’ symptoms and recovery via smartphone, was a one-man operation, and he needed help.
“The rallying point was the accelerator,” says Seiguer, who launched emocha in fall 2013 by licensing technology from the Johns Hopkins University. “It allowed me to quickly recruit a strong team because there’s a lot of excitement generated by joining an accelerator and a solid program.”
back to top 

Please plan to attend and also help spread the word about some exciting technology transfer programs planned for the NIH Research Festival next week:
Monday, September 22nd from 12-2pm
“Careers for scientists in technology transfer & business development” Poster Session I Building 10 – FAES Academic Center Presenter: Steve Ferguson (NIH/OTT)
Monday, September 22nd from 12-2pm
“NIH and the Federal Laboratory Consortium for Technology Transfer” Poster Session I Building 10 – FAES Academic Center Presenters: Steve Ferguson (NIH/OTT) and Gary Jones (Federal Laboratory Consortium)
Monday, September 22nd from 2-4pm
“Commercial Development of my Research: Still More Personal Stories from former NIH Intramural Scientists” Concurrent Symposium Session I Building 10 – FAES Academic Center – Classroom 3
Co-chairs: Steve Ferguson (NIH/OTT) and Todd Chappell (BioHealth Innovation)
The full schedule of events at the NIH Research Festival (September 22-24) can be found at: http://researchfestival.nih.gov/2014/index.shtml
back to top 

The CEO and co-founder of Oculus VR is giving the University of Maryland $31 million – the largest gift in university history – to construct a computer science building.
University officials said late Thursday that alumnus Brendan Iribe’s gift will help build the Brendan Iribe Center for Computer Science and Innovation. Most of the gift will go toward the building, which will be designed for work in virtual reality, augmented reality, computer vision and robotics, and $1 million will establish the Brendan Iribe Scholarship in Computer Science.
back to top 

Harpoon Medical, Inc., a medical device company focused on the development and commercialization of a minimally invasive, beating heart mitral valve repair technology, announced today that the company has successfully raised $3.2M of new capital and expects to complete the round over the coming weeks. The Series A financing was led by Epidarex Capital in order to advance Harpoon’s innovative technology into the clinic and expand the company’s research and development efforts.
“We are very pleased with the results of our Series A financing because it gives Harpoon Medical the resources necessary to advance the technology from an innovative concept into the clinic,” said, Bill Niland, CEO of Harpoon Medical, Inc. and serial healthcare entrepreneur. The round was increased to accommodate strong demand from investors that include the Maryland Venture Fund, the Abell Foundation, medical device executives, doctors and successful business executives in the Maryland area. “When you have the opportunity to put together a high caliber syndicate of investors who can help you with more than just capital you find a way to accommodate the demand.”
back to top 

Brendan Iribe dropped out of the University of Maryland here, but before he did he amassed 227 parking tickets. And he managed to meet two business partners who would help him build the virtual-reality company Oculus VR, which Facebook bought this year for about $2-billion.
One of those parking tickets remains unpaid, but the university is likely to forgive it after Friday, when he gave $31-million to erect a computer-science building. That makes Mr. Iribe, who is 35 years old, the institution’s most generous donor ever.
back to top 

The Johns Hopkins University has entered into a partnership agreement with Google’s Advanced Technology and Projects group aimed at speeding up the development of new technology and moving the resulting products toward the marketplace more quickly.
The agreement will enable ATAP to draw on the expertise of computer scientists and others at Johns Hopkins and approve funds for joint technology projects in as few as 30 days. That turnaround time is much shorter than the period typically required for obtaining grants from government agencies and private organizations.
back to top 

As the Ebola death toll climbs above 2,000, a university researcher is working to develop a vaccine for the virus.
Alan L. Schmaljohn, a microbiology and immunology professor at University of Maryland, Baltimore, has successfully isolated one of the structural sections of the virus, which can be used as a platform for a working vaccine.
back to top 

Pluristem Therapeutics, a company that develops placenta-based cell therapy products, announced that its licensing partner United Therapeutics is advancing the Phase I study of Pluristem’s PLacental eXpanded (PLX-PAD) cells in patients with pulmonary arterial hypertension (PAH).
PLacental eXpanded (PLX) cells are developed by Pluristem to serve as protein delivery platforms that release a mix of proteins for ischemia or inflammation. The treatment technology is also being used to investigate tendon-healing treatments in a preclinical model of tendon injury. The company presented results at the American Academy of Orthopedic Surgeons’ (AAOS) Annual Meeting last March in New Orleans.
back to top 

In the fight against cancer, Johns Hopkins University is invoking a popular attitude these days: go big or go home.
The Johns Hopkins radiation oncology department has teamed up with Toshiba Group to establish the Toshiba Center for Big Data in Healthcare at the Science + Technology Park at the Hopkins campus in Baltimore.
back to top 

A white powdered chemical compound emerged from two University of Maryland School of Medicine laboratories more than 10 years ago with a name destined for oblivion, but a future that now looks promising as a treatment for the most challenging cases of prostate cancer.
Today, VN/124-1 is a drug candidate with a name — galeterone — a pharmaceutical company founded on its potential and a record of strong preliminary results in clinical trials with human patients.
back to top 

Startup Maryland announced support from TEDCO (www.tedco.md) as a full-tour sponsor of the Pitch Across Maryland 2014, the third annual state-wide tour and celebration of entrepreneurship and startup companies.
Starting on September 15 and running through October 3 the Pitch Across Maryland tour will again traverse the state all in the name of celebrating Maryland’s diverse communities of venture building.
“Maryland’s innovation economy is front-and-center in many of the most lucrative industry and innovation categories,” stated Michael Binko, founder of Startup Maryland. “From traditional ‘feds, eds and meds’ (government/education/healthcare/life sciences-bio), to cloud computing, cyber-security, creative/arts, clean energy/green, to autonomous systems, Maryland entrepreneurs are breeding innovation ventures that are disrupting a wide array of industry sectors and TEDCO has consistently been the recognized leader in early-stage resource commitments.”
back to top 

Optum Labs has named the University of Maryland, Baltimore (UMB) as one of the latest partners to join its research collaborative. Led by Eleanor Perfetto, PhD, MS, professor in the Department of Pharmaceutical Health Services Research (PHSR) at the University of Maryland School of Pharmacy, this new partnership will enhance and augment UMB’s existing research and informatics resources with the data, tools, expertise, and infrastructure available at Optum Labs to increase the scope and impact of Alzheimer’s disease and healthy aging research.
“This partnership with Optum Labs enhances UMB’s recognition as a leader in ‘big data’ research,” says Perfetto. “In addition to expanding research opportunities for faculty and students across the University, the partnership increases our competitiveness for grants and contracts from industry, government, and philanthropic organizations. We look forward to combining our expertise and resources with those at Optum Labs to pursue innovative projects that will improve health care delivery and patient outcomes for individuals with Alzheimer’s diseases and other aging-related issues.”
back to top 

Some of the greatest health care innovations have taken decades to reach widespread adoption, adding to the ever-increasing cost of health care.
We are looking for solutions that have already been implemented at least once and have demonstrated effectiveness. This “scale-up” competition seeks to shorten the time frame for innovation dissemination.
back to top 

Say you invent a medical device. A pacemaker. An improved hip implant. A microchip for the brain. Maybe you can change the world, but first you’ll have to get approved.
A new graduate certificate program in the bioengineering department teaches students the ins and outs of gaining Food and Drug Administration approval. This process is necessary to test the safety and efficiency of all medical inventions before they hit the market, but it can take years of expensive research — and disapproval is common, said William Bentley, the bioengineering department chairman.
back to top 

When we go grocery shopping, most of us don’t buy a pallet of one product and survive on that for the rest of our lives. We get our food more regularly, so it’s fresher and more in tune with our tastes of the moment. So why wouldn’t education in the fast-changing STEM fields, look like that too?
That was how Andrew Coy, the executive director of the Digital Harbor Foundation, put it during an informal tech leaders roundtable discussion at City Hall Thursday morning.
back to top 

Today, the University announces the receipt of its largest gift ever by a single donor. It will catapult our top-15 computer science program to even greater national and international pre-eminence. It will spark innovation and entrepreneurship across the campus and catalyze new economic development in the state.
The gift began after a tragedy and will end in a living memorial. It demonstrates the impact of friendship, teamwork, and family—qualities that ultimately benefit our students and faculty.
back to top 

The innovation program designed to move academic findings and translational research into the commercial marketplace, known at Johns Hopkins University as FastForward, is expanding early next year, with a second facility scheduled to open in East Baltimore to provide lab and office space for startups. Some lab space will be available starting in September 2014.
The first accelerator, FastForward Homewood, at the historic Stieff Silver Building near Homewood campus, was opened by the Whiting School of Engineering in June 2013, and today it houses nine startups from across the university. The second incubator, FastForward East at the Rangos Building on North Wolfe Street, will be more closely tied to the Johns Hopkins School of Medicine, the Bloomberg School of Public Health, and the Johns Hopkins School of Nursing, while still allowing scientists and technical experts from a wide background to take advantage of the new facility.
back to top 

Get answers now from experienced entrepreneurs and legal/business professionals on how to build a successful startup company. Receive free and impartial advice, brainstorm business strategies, investigate funding opportunities and learn about the vast resources available to entrepreneurs.
DATE: September 16, 2014
PLACE: Columbus Center, 701 East Pratt Street, Baltimore, Maryland 21202 USA
REGISTER: Schedule an appointment here to guarantee your time slot. Walk-in appointments are also available.
back to top 

There were few surprises for local colleges and universities in the oft-quoted U.S. News and World Report annual rankings released Tuesday.
The Johns Hopkins University maintained its 12th-place position for national universities, falling between Dartmouth and Northwestern. Hopkins is focusing on its undergraduate experience, with a goal of making it among the top 10 in the nation by 2020.
back to top 

nvestors in entrepreneurs have a special in-the-trenches wisdom gained through years of experience. This investor experience is now available to entrepreneurs and prospective early-stage investors through InvestorIQ.org, a free, online curriculum that provides knowledge essential to improving startup investment.
Investor IQ allows startup backers and founders to educate themselves on how investors decide whether to invest at all, where to invest, and how their money will be used and returned. A video series draws on the successes and failures of thousands of angel investors over the last 15 years, delving into five questions early-stage investors should ask themselves before they put money into an entrepreneurial venture.
back to top 

It’s no surprise that social-media campaigns can raise awareness of an issue, but the ALS Ice Bucket Challenge may be unprecedented in its impact on a relatively rare disease. The campaign, in which participants must donate to an ALS cause or take videos of themselves being doused in ice, has gone viral since it began in late June. But it has also generated controversy, with some questioning the attention and flood of cash for a disease that affects a small number of people.
As of Friday, the ALS Association had received $53.3 million since July 29, compared with $2.2 million by that time last year. To put it in perspective, the National Institutes of Health’s yearly budget for ALS research is $40 million. Other ALS charities are also benefitting similarly from the campaign.
back to top 

The Southern Maryland Innovation and Technology (SMIT) initiative has awarded Smartronix and Coherent Technical Services, two locally headquartered technology firms, Pro Memberships to CoFounders Lab.
CoFounders Lab, a OneVest Company, is an online partnering platform of more than 40,000 founders, advisers and interns available to help launch and grow businesses. A Pro Membership provides access to entrepreneurs nation-wide to help individuals connect with the right partner, investment or resource needed to take a business to the next level. SMIT will award 40 Pro Memberships to individuals and companies to encourage technical innovation and commercialization in St. Mary’s County.
back to top 

Over the course of three days at the Mayo Clinic‘s annual Transform conference, featuring scores of healthcare’s top names, several themes stood out. Here’s what I gather were the main points.
1. Economic factors and barriers are just as important in determining health outcomes for the population as a whole. It stands to reason – people with fewer financial resources are often forced to choose between paying for a costly lab test or doctor visit or putting food on the table, among countless other instances and examples.
back to top 

My last post, How Successful People Stay Calm, really struck a nerve (it’s already approaching 1.5 million reads here on LinkedIn). The trick is that managing your emotions is as much about what you won’t do as it is about what you will do.
TalentSmart has tested more than a million people and found that the upper echelons of top performance are filled with people who are high in emotional intelligence (90% of top performers, to be exact). So, I went back to the data to uncover the kinds of things that emotionally intelligent people are careful to avoid in order to keep themselves calm, content, and in control. They consciously avoid these behaviors because they are tempting and easy to fall into if one isn’t careful.
back to top 

There has been a long-running debate in the pharmaceutical industry about the value of being first to market. Companies spend considerable resources seeking to increase the odds of beating their competitors to market and often fret about the commercial disadvantage of being late. In the high-stakes race to market for a novel drug class, companies firmly believe that every month of lead time ahead of a competitor is significant.
It’s not quite that simple. Our analysis of pharma launches confirms a weak first-to-market advantage on average, but with significant nuances dependent on market context. In many instances, the first-mover advantage actually vanishes, particularly when the lead time is short or when the first mover is a small company. This article seeks to identify those situations where first-to-market advantage is strong and those where it does not hold.
back to top 

This is the first in an exciting series of programs designed to keep you current on issues affecting you as a leader of your organization, and provides an opportunity to connect with your colleagues.
On October 7, Gary will discuss the role of disruptive technology in today’s market and how to create an adaptive, decisive, mission-oriented corporate environment that can help you drive your company forward. In addition to leading one of the largest associations of technology companies, Mr. Shapiro is at the helm of the International CES (Consumer Electronics Show), the world’s largest annual innovation event. It unites more than 150,000 retail buyers, distributors, manufacturers, market analysts, importers, exporters, and press from 150 countries.
Join us as Gary shares his passion for innovation and explains what it is like to be at the forefront of a multi-billion dollar industry and what is coming next.
I look forward to seeing you at this terrific event. Click here to register now!
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 15
Deadline

September 15-16
Sheraton Pentagon City

September 15
Various Locations

September 16
UMBC

September 18-19
Baltimore Marriott Inner Harbor at Camden Yards
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Emergent BioSolutions Inc. (EBS) announced today that it has signed a contract with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), to develop a dry formulation of NuThraxTM (Anthrax Vaccine Adsorbed with CPG 7909 Adjuvant), also known as AV7909, the companys next generation anthrax vaccine candidate. This five-year contract, valued at up to $29 million, provides funding for manufacturing and non-clinical activities through the preparation of an Investigational New Drug application to the U.S. Food and Drug Administration. The dry formulation of NuThrax is intended to increase stability of the vaccine candidate at ambient and higher temperatures, with the objective of eliminating the need for cold chain during shipping and storage.
back to top 

MIMETAS, in a consortium with Radboudumc and FHNW, has received 1.6 million USD funding for development of a kidney-on-a-chip for toxicological applications. A panel of experts from GlaxoSmithKline, Pfizer, Roche, NC3Rs and renowned academic institutions selected MIMETAS’ solution from a strong line-up of competing technologies.
The funding is awarded in the context of the NephroTube Crack-it Challenge to support development of a microfluidic renal model predicting renal toxicity during pre-clinical development. In collaboration with the Radboudumc Pharmacology-Toxicology Department and the Swiss FHNW, MIMETAS will use the funds to develop a high-throughput kidney-on-a-chip model by combining its OrganoPlate™ 3D-culturing technology with the human renal cell line ciPTEC™, analyzed and validated in three separate sites. The resulting model will be used to detect renal tubular injury observed in drug-induced nephrotoxicity. The model’s early prediction of nephrotoxicity will help to reduce animal experiments.
back to top 

The National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) in Bethesda, Maryland is seeking a dynamic, innovative and accomplished biomedical/biotechnology executive with demonstrated scientific and entrepreneurial expertise to provide strategic vision and leadership for the Office of Translational Alliances and Coordination (OTAC). The OTAC is charged with developing, implementing and leading translational research programs that create recognizable commercial value for discoveries and innovations during their gestational stages and facilitating their ultimate translation into new diagnostics, devices, therapeutics and tools. The Office is also charged with identifying emerging areas of translational opportunities, serving as a focal point for extramural researchers for information on NHLBI-wide small business technology development opportunities.
The OTAC Director is an expert in entrepreneurism and technology advancement with the vision and unique skill set required to recognize commercial value in very early stage technologies, guide their scientific and business oriented risk-mitigating development and the capital acquisition activities required for their ultimate customer/patient deployment. These skills, gained through experience in both the academic research and the start-up/small business or industry sector, will provide the OTAC Director the capability to oversee and effectively manage existing NHLBI translational research technology development programs and the development of new programs and initiatives required to identify and advance innovations and technology platforms for research, diagnosis, treatment, control and prevention of cardiovascular, lung, blood and sleep disorders.
back to top 

WHEN: Thursday, Sept 25th, 2014 From 3:00 to 5:00 PM
WHERE: One MedImmune Way Gaithersburg MD 20878
BioBuzz Double Helix Sponsor MedImmune is hosting a special BioNetworking event for Post-Docs and Professional Scientists on September 25th at their headquarters in Gaithersburg.
This event will include music, lawn games, hors d’oeuvres, drinks and a scientific “Speed Networking” session and is open to Post-Docs & Professional Scientists who currently work in the Therapeutic Areas of:
* Oncology
* Respiratory, Inflammation & Autoimmunity
* Cardiovascular & Metabolic Diseases
* Infectious Diseases & Vaccines
* Related scientific disciplines
To RSVP: email info to MedIPartneringStrategy@MedImmune.com Sign up by 9/12 and Provide your:
Name
Company
Phone
Therapeutic Area
Let them know you learned about this event through BioBuzz!
This event is for Post-Docs & Professional Scientists
back to top 

The I2C Conference is a full-day event to help you get your technology-based small business on the fast-track! Three in-depth panels on Innovation, Commercialization and Financing Speakers include: GlycoMimetics, MedImmune, Johns Hopkins University, Naval Research Labs, Brain Sentry, Mindoula, SmartSenseCom, TEDCO, Maryland Venture Fund, and the exit strategy team from Amplimmune. Enjoy lunch…
back to top 

Are you an entrepreneur ready to pitch your company? Register for your chance to film a three-minute video pitch to be seen online by VCs and angel investors and be voted on by fans around Maryland!
The Montgomery County Department of Economic Development and the Montgomery County Chamber of Commerce have partnered to host Montgomery County’s Pitch Across Maryland stops on October 1, 2014. Applications for the Montgomery County Pitch stops are due September 15!
back to top 

Swiss drugmaker Roche Holding AG said on Monday the European Union has approved the use of its drug RoActemra in patients with early-stage rheumatoid arthritis.
Roche said the European Commission has backed RoActemra as a treatment for patients with severe, active and progressive rheumatoid arthritis who have previously not been treated with methotrexate.
back to top 

When British pharmaceutical giant GlaxoSmithKline announced in October 2012 that it planned to make detailed data from its clinical trials widely available to researchers outside its own walls, the scientific community was stunned. For a company that spends $6.5 billion a year on research and development, it was a sharp turn away from the system of data secrecy that had made it one of the world’s largest drug companies, with 2013 sales of $43.6 billion.
The announcement came a few months after the company pled guilty to misdemeanor charges in the U.S. that it had marketed drugs for unapproved uses, based on improperly reported clinical trial data, and failed to report safety data on another drug later shown to raise the risk of heart attacks. Given the timing, many wondered if GSK’s move was more about rehabilitating its image than embracing data transparency.
back to top 
The 2014 International Biotech Summit Committee invite BioHealth Innovation (BHI) and Montgomery County to attend the three co-located international conferences that will be held on Sep 9 -12 in Shenzhen, China:
- 2014 International Biotech Summit (September 10, 2014);
- The 9th International Conference on Genomics (ICG-9) (September 11th-12th, 2014);
- Shenzhen International Biotech & Health Industry Expo 2014 (SIBHIE, September 10th-12th, 2014).
Ms. Lily Qi, Director of Special Projects for Montgomery County Executive Office who was involved in establishing BHI and a native of Shanghai, is going to speak on behalf of the County and BHI for the forum, “Innovation in Policy and Regulation for Advancing Healthcare Industry.” BHI and the County are also invited as an exhibitor for the SIBHIE Expo.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Implementation of the NIH Genomic Data Sharing Policy for NIH Grant Applications and Awards
- NIH Genomic Data Sharing Policy
- Public Comments on Proposed Guidance Regarding Significant Changes to Ongoing Animal Activities
- Guidance on Significant Changes to Animal Activities
- Notice of National Biosafety Stewardship Month and Health and Safety Requirements for NIH Grantees
- eRA Commons Username Required for Sponsor in Individual Fellowship Grant Applications to NIH and AHRQ
- NIAMS Policy for Submission of Applications Containing Clinical Trials
- (NOT-AR-14-021)
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
Requests for Applications:
- Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Centers (U54)
- (RFA-AR-15-002)
- National Institute of Arthritis and Musculoskeletal and Skin Diseases
- National Heart, Lung, and Blood Institute
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Neurological Disorders and Stroke
- Application Receipt Date(s): November 28, 2014
back to top 

Maryland Industrial Partnerships (MIPS) Funding for Technology Product Development
MIPS provides funding, matched by participating companies, for university research projects that help companies develop new technology products.
Benefits to Maryland Companies
- Cost-effective research with world-class university faculty
- Access to university students, state-of-the-art facilities, laboratories and equipment
- Non-dilutive, non-debt funding for research
- Opportunity to work directly with talented students—potential future hires
- Rapid proposal evaluations—MIPS notifies award winners within 60 days of the proposal deadline
Benefits to Maryland Faculty
- Research translates directly to new product development
- Potential for published papers and improved university facilities
- Students gain valuable experience working on commercial technologies
Visit www.mips.umd.edu or call 301.405.3891301.405.3891 for details.
APPLICATIONS ARE DUE BY OCTOBER 15, 2014!
back to top 

SAVE THE DATE: THURSDAY, NOVEMBER 20, 2014
The MCCC Business Awards Dinner Committee and Chair of the Board Lisa Cines, CPA of Dixon Hughes Goodman LLP invite you to join in the celebration of those who make our economy and community thrive. This annual sold-out event attracts 700+ guests including award winners, sponsors, business leaders, elected and government officials and the media. Join us for a great evening of Meaningful Connections, Commerce and Celebration.
back to top 

A lot has been written lately about innovation, or the lack thereof, in the world of biopharma. One question that often gets asked: which countries lead the way in creating new medicines? Many people think that drugs originate in the nation where the companies that produce them are headquartered. The truth, however, is much more complicated. Given that multi-national firms market the majority of medicines, figuring out where each one of their drugs originated requires digging through some extensive data vaults. A proper analysis requires the examination of company histories, free market deal making, and in some cases government interventions. Consider the following examples:
back to top 

Have you always wondered what type of scientific researcher you are? Take the quiz to find out.
back to top 

This Monday September 1st marked the beginning of when investors with Angel Venture Forum D.C. started looking at submissions from companies for the Nov. Showcase.
AVF screeners do start reviewing plans as soon as submissions are made and some companies who have submitted to date have already received assistance and invitations to angel groups. Since we typically get a large number of applications and it does take the screeners a while to get through them all. Thus, the sooner an application the better.
The initial screening will occur through Sept. 15th. So you may still make submissions and corrections on applications up until Sept. 15th if needed. However, it is recommended that you complete your submission as soon as possible so that you get in the queue.
To submit an application – no later than Sept. 15th – visit www.angelventureforum.com. For any questions, please feel free to contact Mike Gildea, AVF COO at mgildea@braingainllc.com or 814-451-1151.
back to top 

The Food and Drug Administration on Thursday approved the first of an eagerly awaited new class of cancer drugs that unleashes the body’s immune system to fight tumors.
The drug, which Merck will sell under the name Keytruda, was approved for patients with advanced melanoma who have exhausted other therapies.
back to top 

GETTING IDEAS TO PATIENTS: THE SOPE FOUNDATION CROWDFUNDING CAMPAIGN NEEDS YOUR SUPPORT FOR EDUCATIONAL PROGRAMS
To provide SoPE members with educational programs, events and more, we need your support. The SoPE Foundation has started a crowdfunding* campaign —Getting Ideas to Patients—to raise $250,000 by the end of 2014 for educational programs and events. The SoPE Foundation is the sole funder for these activities, such as the Blakely Visiting Lectureship Series named posthumously for one of our co-founders, and support for biomedical and health innovators applying for the Innovation Scholars Program. SoPE’s operates on a low-cost membership model to encourage enrollment. With your financial contribution, we will have the funding to do what we set out to do. Every little bit helps, and every larger bit moves us closer to our goal.
back to top 

Dubbed as the most severe and deadly outbreak, the Ebola menace in West Africa has caught the attention of GlaxoSmithKline plc (ADR) (NYSE:GSK) and National institute of health. CNBC’s Meg Tirrell reports that NIH and GlaxoSmithKline have partnered to develop a vaccine that is said to have had immense success in primates, but yet to be tested in Humans.
The studies are to be used to ascertain whether the vaccine is safe and also its ability to prompt an immune response, able to combat the Ebola virus. No humans are to be infected’ with the Ebola virus during the course of the study.
back to top 

Two Washington entrepreneurs with a history of building successful companies have teamed with a local businessman to start a venture capital fund focused on seed stage companies focused on applications that run on mobile devices.
The fund, called Kiwi Venture Partners I , has raised $2.5 million from 24 limited partners, including 12 from the Washington area. The LP investments range from $25,000 to $500,000.
back to top 

According to CB Insights data, funding to digital health/health IT companies has already topped $2.2B in the first half of 2014.
With the space becoming increasingly crowded and well-funded, one effective way innovation trackers can understand which areas (and likely companies) will play a key role in future of the digital health space is by following the smart money.
back to top 

Anne Arundel Medical Center is looking to get in on technology commercialization, a game more typically played at research hospitals and universities.
The James and Sylvia Earl Simulation to Advance Innovation and Learning (SAIL) Center is developing a plan to bring together doctors and the local business community to commercialize technology that would benefit the hospital.
back to top 
Legal 500, considered one of the most comprehensive worldwide qualitative guides available on legal services providers, recently announced its 2014 rankings. Venable’s national Corporate Group continued to earn high marks in the latest edition leading the way with a Tier 1 ranking in the M&A: Middle-Market (Sub-$500m) category. The group also received high marks in the Technology: Outsourcing, Technology: Transactions and Real Estate Investment Trusts (REITs) categories.
Corporate Practice Co-Chair Charles J. Morton, Jr. and partner William T. Russell received special recognition as Leading Lawyers for M&A: Middle Market (Sub $500m) Technology: Transactions respectively. Leading Lawyers are selected from all the nationally recommended attorneys in a particular category and represent those few individuals, who, in the view of their peers, represent the highest standards of their practice. This is Mr. Morton’s third year as a Leading Lawyer and Mr. Russell’s fourth. Mr. Morton was one of 23 attorneys nationwide named a Leading Lawyer in M&A: Middle Market (Sub $500m). Mr. Russell was one of only 12 attorneys nationwide named a Leading Lawyer in Technology: Transactions.
back to top 

A group of UC Berkeley students launched what they call the first-ever student-run health tech incubator Thursday at the office of Downtown Berkeley’s Skydeck, which fosters campus startups.
The incubator, Catalyst@Berkeley, aims to provide a framework for students to bring viable prototypes to the market and open doors of entrepreneurship to undergraduate students interested in health care innovation.
back to top 

The Life Sciences Discovery Fund (LSDF) today announced nearly $750,000 in Proof of Concept grants to Washington state for-profit and non-profit organizations to promote translation of health-related technologies from the laboratory to the commercial marketplace. Also announced was nearly $56,000 in supplemental funding to an existing grant to increase the commercial potential of a drug to protect hearing in patients taking certain antibiotics. (See Backgrounder Information.)
The LSDF Board of Trustees selected the awardees following review of proposals for scientific and technical merit, commercial potential, and health and economic benefits to Washington.
back to top 

Friday, November 14, 2014
Encounter the hottest trends and innovations in Health IT as well as investment at the “eHealth Venture Summit”. This event at the MEDICA Health IT Forum showcases new companies, offers valuable insights and shall foster strategic partnerships and investment opportunities crucial to bringing innovative products to market. The event will feature seasoned industry experts from leading companies and funding specialists. The conference is jointly organized by Prof. Talya Miron-Shatz, CEO of CureMyWay, and a faculty member at the Ono Academic College, and Dr. Stefan Becker of the Institute for Drug Safety, University Hospital Essen.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

September 9
UMD Mtech

September 10-12
Shenzhen Convention & Exhibition Center

September 10
Shenzhen Convention & Exhibition Center

September 10-12
Washington, DC | San Francisco, CA

September 10
Germantown Innovation Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
Legal 500, considered one of the most comprehensive worldwide qualitative guides available on legal services providers, recently announced its 2014 rankings. Venable’s national Corporate Group continued to earn high marks in the latest edition leading the way with a Tier 1 ranking in the M&A: Middle-Market (Sub-$500m) category. The group also received high marks in the Technology: Outsourcing, Technology: Transactions and Real Estate Investment Trusts (REITs) categories.
Corporate Practice Co-Chair Charles J. Morton, Jr. and partner William T. Russell received special recognition as Leading Lawyers for M&A: Middle Market (Sub $500m) Technology: Transactions respectively. Leading Lawyers are selected from all the nationally recommended attorneys in a particular category and represent those few individuals, who, in the view of their peers, represent the highest standards of their practice. This is Mr. Morton’s third year as a Leading Lawyer and Mr. Russell’s fourth. Mr. Morton was one of 23 attorneys nationwide named a Leading Lawyer in M&A: Middle Market (Sub $500m). Mr. Russell was one of only 12 attorneys nationwide named a Leading Lawyer in Technology: Transactions.
back to top 

GlaxoSmithKline (GSK) is one of the world’s leading research-based pharmaceutical and healthcare companies, has announced that it will freeze the prices of its vaccines for five years for developing countries that graduate from GAVI Alliance support.
By committing to offer GAVI Alliance prices for vaccines against pneumonia, diarrhoea and cervical cancer, GSK will support developing country governments as they transition to financing the full cost of their local vaccination programmes.
back to top 

The crossover round is alive and well. Just a few days after Dermira put out the word that a consortium of backers had come up with a $51 million C round, the company rolled out a $75 million IPO. And the news, along with a fresh, $86 million filing from Rhythm Pharmaceuticals, helps set the stage for a new round of fall biotech IPOs that will once again test investors’ appetite for risk.
First, let’s look at Redwood City, CA-based Dermira. Focused on skin ailments, the biotech is partnered with UCB on the development of Cimzia–already on the market–for psoriasis, a field that will soon be packed with a host of contenders from the likes of Novartis ($NVS) and Eli Lilly ($LLY). The biotech has laid plans for a Phase III psoriasis trial in 2015 as it pursues further work on a new therapy for “hyperhidrosis,” or excessive sweating, in the armpits. It’s in a Phase IIb study and a successful outcome would set the stage for a late-stage program. There’s also an acne treatment in early mid-stage studies.
back to top 

The Maryland Department of Business and Economic Development says its Maryland Venture Fund has made follow-on investments in two Maryland startups totaling $700,000.
Bethesda-based KoolSpan, a mobile security encryption company, received $400,000. BrainScope, also in Bethesda, got $300,000. BrainScope is developing neurotechnology for quickly assessing traumatic brain injury.
back to top 

Emergent BioSolutions is looking to fill a position for a senior scientist position.
If hired, the senior scientist will be responsible for vaccine potency and assay development at Emergent BioSolutions.
Key responsibilities of the position include the design and execution of experimental protocols for developing and qualifying animal-based potency assays, conducting in vitro potency assay development and leading vaccine product stability studies with the company’s Potency Assay Group.
back to top 

Steve Blank, a serial entrepreneur, author and professor at Stanford University, developed the curriculum for the National Science foundation’s business boot camp for scientists before the NIH adopted it for scientists in the SBIR grant program. At a fireside chat at New York University Langone Medical Center hosted by NYC Bio this week, Blank talked about a new investment group called M34 Capital that will invest in technologies seeded by federally funded research, particularly graduates of I-Corps boot camps.
Led by CEO Errol Arkilic, founding and lead program director for the NSF I-Corps program, the Berkeley-based company was co-founded by Arkilic, Blank, Jim Hornthal, and Tom Baruch, according to its website. Its seed investment spans from $50,000 to $250,000. “We want to be the first external capital invested, and work closely with the founding team to help refine their business model and create the framework to systematically demonstrate its full potential,” according to the website. “We believe that the best entrepreneurs have the ability to blend technical insight with market-based feedback, allowing their innovations to mature into a successful company.”
back to top 

Howard University Hospital will receive $11 million in federal grants toward developing new therapeutics for the Ebola virus, Howard officials said.
At least $2 million from the National Institutes of Health will go toward developing a new drug to target a particular protein of the Ebola virus. As well, $2 million will go toward work at the hospital researching HIV resistance in people with sickle cell disease, officials said.
back to top 

BHC Center of Financial & Investment Business (“BHC”), an Atlanta based affiliate of Bao Hao Investment Management Co., Ltd. of Shanghai, has announced the first of what it expects to be several equity investments in 20/20 GeneSystems, Inc. (“20/20”). In making the investment BHC is seeking to help advance the introduction in China of 20/20’s blood test for the early detection of lung cancer (see www.BloodTestforLungCancer.com).
20/20 currently produces and markets this innovative blood test for the early detection of lung cancer in the United States. Primary care physicians employ the blood test to help detect lung cancer in patients who are smokers and former smokers. It is known as “PAULAs test.”
back to top 

With a massive influx of new funding, Frederick pharmaceutical company Vaccinogen Inc. is preparing to make big moves that leaders hope will put it on the map.
Vaccinogen announced Aug. 25 it had secured $80 million in funding from Stockholm investors and closed the first $10 million tranche of the funding. The funding will support late-stage clinical studies needed to make Vaccinogen’s OncoVAX commercially available. The drug is designed to reduce recurrence of colon cancer.
back to top 

ATCC, the premier global biological materials resource and standards organization, and bioMérieux, a world leader in the field of in vitro diagnostics, announced today their collaboration to expand and solidify the bioMérieux VITEK® MS microbial identification database for the industrial market. Recently, ATCC acquired the bioMérieux VITEK® MS system to make this innovative MALDI TOF technology a fundamental component of their authentication and quality control processes.
Strengthening the database with additional strains continues to increase the robustness of the VITEK® MS micro-organisms library while increasing the size and enhancing the utility for customers as a solid quality assurance tool.
back to top 

Venture capital (VC) funding for the Life Sciences sector, which includes Biotechnology and Medical Devices, reached $2.5 billion in 195 deals for the second quarter of 2014, according to a new report, “Biotech soars to record high,” which includes data from the MoneyTree™ Report from PricewaterhouseCoopers (PwC) LLP and the National Venture Capital Association (NVCA), based on data provided by Thomson Reuters. While this quarter was the highest Life Sciences investment since the second quarter 2007, it was also the strongest second quarter since 1995, which is the earliest data recorded by the MoneyTree.
back to top 

The National Institutes of Health is challenging science innovators to compete for prizes totaling up to $500,000, by developing new ways to track the health status of a single cell in complex tissue over time. The NIH Follow that Cell Challenge seeks tools that would, for example, monitor a cell in the process of becoming cancerous, detect changes due to a disease-causing virus, or track how a cell responds to treatment.
back to top 

A lot has been written lately about innovation, or the lack thereof, in the world of biopharma. One question that often gets asked: which countries lead the way in creating new medicines? Many people think that drugs originate in the nation where the companies that produce them are headquartered. The truth, however, is much more complicated. Given that multi-national firms market the majority of medicines, figuring out where each one of their drugs originated requires digging through some extensive data vaults. A proper analysis requires the examination of company histories, free market deal making, and in some cases government interventions. Consider the following examples:
back to top 

Student consultants at the University of Maryland’s Robert H. Smith School of Business often find their target audience staring at them in the mirror when they do market research for nonprofit organizations.
ChangeTheWorld.org, a pro bono consulting program launched at the school’s Center for Social Value Creation in 2006, reports an increased interest among nonprofit organizations in the Millennial Generation as the population segment grows up and goes to college. “Nonprofit organizations want to unleash the potential of this generation, and they are coming straight to the source for insights at the Smith School and other partner universities,” said Pammi Bhullar, program manager at the Center for Social Value Creation and ChangeTheWorld.org. “Essentially, they are asking college students to gather market intelligence on themselves.”
back to top 

A candidate Ebola vaccine could be given to healthy volunteers in the United Kingdom, The Gambia, and Mali as early as September, as part of a series of safety trials of potential vaccines aimed at preventing the disease that has killed more than 1,400 people in the current outbreak in West Africa.
Human trials of this candidate vaccine, being co-developed by the National Institutes of Health (NIH) and GlaxoSmithKline (GSK), are to be accelerated with funding from an international consortium in response to the Ebola epidemic, which the World Health Organization (WHO) recently declared a public health emergency of international concern.
back to top 

GlaxoSmithKline’s experimental Ebola vaccine could be tested on humans in the UK, US, the Gambia and Mali in the next few weeks, in a race to contain the deadly virus that has claimed more than 1,500 lives in west Africa.
The news came as the World Health Organisation (WHO) warned that the Ebola epidemic could eventually exceed 20,000 cases. Bruce Aylward, WHO’s assistant director-general for emergency operations, said: “This far outstrips any historic Ebola outbreak in numbers. The largest outbreak in the past was about 400 cases.”
back to top 

Mayor Stephanie Rawlings-Blake on Thursday appointed six new members to the Baltimore Development Corp. board, an announcement that comes as Councilman Bill Cole takes over as president of the agency.
The six new members are mostly long-standing vacancies on the 19-member board. One of the appointments fills a seat opened after city Finance Director Harry Black announced last month that he was leaving his job to become city manager in Cincinnati.
back to top 

Scientists who use government money to conduct genomic research will now be required to quickly share the data they gather under a policy announced on Wednesday by the National Institutes of Health.
The data-sharing policy, which will take effect with grants awarded in January, will give agency-financed researchers six months to load any genomic data they collect—from human or nonhuman subjects—into a government-established database or a recognized alternative.
back to top 

Have you always wondered what type of scientific researcher you are? Take the quiz to find out.
back to top 

Baltimore is going to be OK, as long as it has its big name medical centers.
That’s the big takeaway from a new report by Moody’s Investors Service that looked at the economic impact large medical and education systems have on the cities where they are based.
back to top 
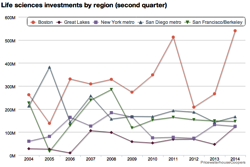
Venture capital continued its love affair with biotechnology in the second quarter, with investment in biotech reaching record levels.
Venture capitalists invested $1.84 billion in 122 biotech deals in the period, according to a MoneyTree report released Monday by PricewaterhouseCoopers and the National Venture Capital Association, using Thomson Reuters data.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Notice to Extend the Expiration Date of PAR-11-315 “Systems Science and Health in the Behavioral and Social Sciences (R21)”
- Notice to Extend the Expiration Date of PAR-11-314 “Systems Science and Health in the Behavioral and Social Sciences (R01)”
- Notice to Extend the Response Date for NOT-HL-14-028 “Request for Information (RFI): Collaborative Translational Research Consortium to Develop T4 Translation of Evidence-based Interventions”
- Notice of NLM’s Participation in PAR-13-358 “Opportunities for Collaborative Research at the NIH Clinical Center (U01)”
- Prize Competition: Challenges in Single Cell Analysis
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

On September 14, 2007, Lorenz Diesbergen, age 44, stepped off a commuter train in downtown Chicago and began his daily walk to work in the Chicago Loop. As he crossed the bridge over the Chicago River, his heart’s normal rhythm suddenly deteriorated into an uncoordinated frenzy of useless fibrillations. He may have managed a few more steps—we don’t know—before he pitched forward and fell face-first onto the sidewalk. Paramedics were on the scene within minutes, but efforts at resuscitation proved futile. He left behind a wife and four children.
back to top 

Bayer HealthCare appears to be the first pharmaceutical company that started its own accelerator. The company that made €18.924 million in turnover in 2013 “understands that innovation and healthcare cannot only be driven by the industry but also needs creativity.” This was announced by Christian Ullrich, Head of Marketing & Sales IT at Bayer HealthCare yesterday.
Bayer has partnered with universities and smaller companies before and started Grants4Apps, a crowdsourcing initiative last year. This year, it was turned into an accelerator.
back to top 

Tue, Sep 9, 2:00 PM – 3:00 PM
Hosted by NHLBI
In this Hangout, we will discuss the basic concepts of medical device regulation in the U.S., what is considered significant risk versus non-significant risk, and how the level of risk factors into the regulation of new product development.
back to top 

Get on the Bus!
Are you an entrepreneur ready to pitch your company? Register for your chance to film a 3-minute video pitch that will be seen online by VC, angel investors and voted on by fans around Maryland!
The Montgomery County Department of Economic Development and the Montgomery County Chamber of Commerce are hosting two Pitch Across Maryland stops in Montgomery County on Wednesday, October 1. Learn More Want to get on the bus and film your pitch? Apply Here.
The Montgomery County application deadline is Monday, September 15.
back to top 

Tuesday, December 2, 2014 from 7:00 AM to 7:00 PM (EST)
The 7th Annual Maryland Stem Cell Research Symposium, hosted by the Maryland Stem Cell Research Commission and Montgomery County will be held on Tuesday, December 2, 2014 at the Silver Spring Civic Center, Silver Spring MD. The Symposium is the Maryland Stem Cell Research Fund premier event that delivers comprehensive scientific talks, poster presentations, and networking time, enabling cell therapy basic research and technologies from the lab to pre-clinical and to commercialization. With a powerful line-up of speakers and many opportunities for you to present your work in concurrent or poster presentations, the Symposium will follow the format and style of previous meetings with additional networking time and an intimate environment.
Abstract Submission:
The Maryland Stem Cell Research Fund (MSCRF) is now accepting abstract submissions for poster and oral presentations for the 7th Annual Maryland Stem Cell Research Symposium. We encourage you to take full advantage of this opportunity to share your research results with our community. Multiple abstract submissions are welcomed.
ABSTRACT SUBMISSION DEADLINE: Wednesday, September 10, 2014
Please Click Here to submit your abstract today!
back to top 

The National Science Foundation (NSF) announced today that it will provide a multi-million dollar grant to create a hub of innovation that unites public and private institutions throughout Southern California, headquartered at and administered by the University of Southern California, the California Institute of Technology (Caltech), and the University of California Los Angeles.
The new center is part of the NSF Innovation Corps, or “I-Corps,” initiative, which is aimed at fostering innovation throughout the U.S. by encouraging the translation of ideas and research beyond the laboratory to create social and economic impact. The announcement cements the position of Southern California as a crucial focal point of technology entrepreneurship in the country.
back to top 

Too much fat weighs down not just your body, but also your brain.
harms most organs in the body, and new research suggests the brain is no exception. What’s more, the researchers found that getting rid of excess fat actually improves brain function, reversing the ill effects of the extra weight. The new study, which focused on people who underwent bariatric surgery, found that the procedure had positive effects on the brain, but other research has shown that less invasive weight loss strategies, like exercise, can also reverse brain damage thought to be related to body fat.
back to top 

Johnson & Johnson and Sanofi are using IBM Watson’s computer brain/big data cruncher to support research and development. It will be used to identify new applications for drugs that have already been developed and to leaf through scientific papers that detail clinical trial outcomes, according to a statement from IBM. The partnerships follow a new development in Watson’s evolution that help it visually uncover patterns and pinpoint connections in related data to accelerate the discovery process and advance science research.
“Watson now has the ability to understand the language of chemistry, biology, legal and intellectual property, giving scientists the ability to make connections with data that others don’t see, which can lead to rapid breakthrough in discoveries,” the statement said.
back to top 

Every day our DNA breaks a little. Special enzymes keep our genome intact while we’re alive, but after death, once the oxygen runs out, there is no more repair. Chemical damage accumulates, and decomposition brings its own kind of collapse: membranes dissolve, enzymes leak, and bacteria multiply. How long until DNA disappears altogether? Since the delicate molecule was discovered, most scientists had assumed that the DNA of the dead was rapidly and irretrievably lost. When Svante Pääbo, now the director of the Max Planck Institute for Evolutionary Anthropology in Germany, first considered the question more than three decades ago, he dared to wonder if it might last beyond a few days or weeks. But Pääbo and other scientists have now shown that if only a few of the trillions of cells in a body escape destruction, a genome may survive for tens of thousands of years.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

The 2014 Steering Committee of the Montgomery County Executive Hispanic Gala (MCEHG) announces the first of three awards to be presented during the gala celebration on September 18, 2014 to take place at The Fillmore Silver Spring.
University of Maryland President, Dr. Wallace D. Loh, will be the 2014 recipient of the “Advocate of the Year Award”.
back to top 

Location: MD, Gaithersburg – Corporate Headquarters
This will be a highly visible position dedicated to ensuring MedImmune further builds its collaboration efforts with governments, universities and non-profit organizations. The primary objective of this role will be to further entrench MedImmune in key biotech clusters to gain access to leading science and technology, enhance MedImmune’s reputation and create operational flexibility. The role will entail scouting for cross-therapeutic scientific collaboration opportunities, negotiating deals, working closely with internal scientists, event planning and strategic planning and analysis.
back to top 

MorphoSys AG (MOR) MPSYY, and Emergent BioSolutions Inc. EBS, today announced an agreement for the joint development and commercialization of ES414. The compound, to be renamed MOR209/ES414, is an anti-PSMA/anti-CD3 bi-specific antibody targeting prostate cancer, which was developed by Emergent using its proprietary ADAPTIRTM (modular protein technology) platform. Preclinical in vitro and in vivo studies have shown that MOR209/ES414 redirects T-cell cytotoxicity towards prostate cancer cells expressing Prostate Specific Membrane Antigen (PSMA), an antigen commonly found on such cells.
back to top 
The 2014 International Biotech Summit Committee invite BioHealth Innovation (BHI) and Montgomery County to attend the three co-located international conferences that will be held on Sep 9 -12 in Shenzhen, China:
- 2014 International Biotech Summit (September 10, 2014);
- The 9th International Conference on Genomics (ICG-9) (September 11th-12th, 2014);
- Shenzhen International Biotech & Health Industry Expo 2014 (SIBHIE, September 10th-12th, 2014).
Ms. Lily Qi, Director of Special Projects for Montgomery County Executive Office who was involved in establishing BHI and a native of Shanghai, is going to speak on behalf of the County and BHI for the forum, “Innovation in Policy and Regulation for Advancing Healthcare Industry.” BHI and the County are also invited as an exhibitor for the SIBHIE Expo.
back to top 

BioHealth Innovation, Inc. (BHI), a Montgomery County innovation intermediary which translates market-relevant research into commercial success by bringing together among other things management, funding, and markets, is seeking an experienced life science professional with entrepreneurial experience to serve as an Entrepreneur-in-Residence (EIR).
The EIR will lead in the evaluation of early-stage technologies and corporations with a priority focus on those associated with Funding Partners and those that best fit with the strategic direction as set by BHI’s CEO and Director of EIR Programs. The EIR will perform any reasonable task which will forward the goals of BHI. Further specifics and responsibilities include:
back to top 

A team of Biomedical Engineering undergraduate students from Johns Hopkins recently won the 2014 Design by Biomedical Undergraduate Teams (DEBUT) Challenge for developing a device that aims to help surgeons safely and accurately place screws during spinal fusion procedures and surgeries for spinal abnormalities.
The AccuSpine pedicle probe was designed by eight undergraduates: Clay Andrews, Eric Xie, Adarsha Malla, Bradley Isaacs, Anvesh Annadanam, Erica Schwarz, Ravi Gaddipati, and Luis Herrera. Their project began in July 2013 as part of a Center for Bioengineering Innovation and Design design team class. Their goal was to address a clinical need for an effective guidance system for the safe and accurate placement of screws.
back to top 

DATE: September 10, 2014 – 3:30 – 5:00 PM
LOCATION: Germantown Innovation Center, 20271 Goldenrod Lane, 2nd Floor, Germantown, MD 20874
As a federal agency, the Department of Energy is creative and innovative. Unlike other federal laboratories, each contractor-operated laboratory manages its own tech transfer agreements. Learn how to navigate these various opportunities and identify the best way to gain access to the technologies and partnerships to enhance your companies growth.
David E. Koegel
David Koegel is the senior technoogy transfer advisor in the Office of Science (SC) at the Department of Energy. A member of the Department’s Technology Transfer Policy Board, his experience in technology transfer goes back to the early 90’s when he was the Office’s Program Manager for American Textile partnership in the Laboratory Technology Transfer Program, a technology transfer collaboration among members of the integrated textile industry, the DOE national laboratories, a number of universities, and several research/education/technology transfer organizations. A member of the National Advisory Council of the Federal Laboratory Consortium, David has been the Department’s representative to the FLC for many years, as well as their representative to the federal Interagency Working Group on Technology Transfer. One of his significant duties involves the annual appraisal of the ten Office of Science national laboratories and is the primary point of contact for the various programs and organizations that use the laboratories’ personnel and facilities. Previously, he had been the Department’s Senior Technology Analyst in the SBIR/STTR Program, the Office of Science Small Business Program Manager, as well as the Program Manager for the Advanced Energy Projects program.
David joined the Department after spending several years at the Pentagon in the Office of the Assistant Secretary of the Army for Research, Development, and Acquisition, most notably during Desert Shield/Desert Storm, acting as a liaison to the Army’s laboratory system. Prior to this, he conducted research at the Army’s Night Vision Laboratory using metal organic chemical vapor deposition for the development of advanced infrared imaging devices.
David earned a B.S. in Chemical Engineering and an M.S. in Materials Science Engineering.
back to top 

The Maryland Technology Development Corp. is launching a new investment fund dedicated to cyber security.
The fund will make investments of up to $100,000 in companies that are developing and commercializing new cyber security projects.
back to top 

Roche Holding AG said Sunday it would pay $8.3 billion for a California biotech firm that has yet to turn a profit on a new drug to treat a deadly lung disease—the latest gamble by a pharmaceutical giant to buy its way into a lucrative corner of the industry.
The takeover would allow the Swiss company to expand its presence in the treatment of respiratory disorders, one of the world’s biggest drug markets. Roche’s offer of $74 a share represents a 38% premium over InterMune’s closing share price on Friday of $53.80, and a 63% premium before takeover speculation surrounding the biotech started circulating this month.
back to top 

Roche has just announced the acquisition of the U.S. biotech company InterMune for $8.3 billion. The driver for Roche was to gain access to InterMune’s drug, pirfenidone (trade name, Esbriet), which is already approved in Canada and Europe for idiopathic pulmonary fibrosis (IPF). IPF is a deadly lung-scarring disease which affects 50,000 – 70,000 people in the U.S. and 80,000 to 110,000 in Europe. While still under review by U.S. regulatory authorities, Roche’s acquisition signals its belief that pirfenidone will also gain U.S. approval. How big a product can pirfenidone be? Consensus forecasts compiled by Thomson Reuters Pharma peg annual sales at greater than $1 billion in 2019 – a nice addition to Roche’s stable of pulmonary compounds including Xolair (asthma) and Pulmozyme (cystic fibrosis).
back to top 

The Daily Record announced Thursday its 2014 Innovators of the Year, celebrating Marylanders and Maryland-based companies for their innovative spirit.
Twenty-eight innovators will be celebrated during the awards event on Oct. 15 at the American Visionary Art Museum, at 800 Key Highway in Baltimore.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
Updated Forms and Instruction Clarification for Re-entry (PA-12-150) and Diversity (PA-12-149) Administrative Supplements
(NOT-OD-14-118) National Institutes of Health
Small Business Innovation Research (SBIR) Program Contract Solicitation (PHS 2015-1) Now Available
(NOT-OD-14-120) Office of the Director, NIH
Notice of Participation of NLM in PAR-13-357 “Pre-application: Opportunities for Collaborative Research at the NIH Clinical Center (X02)”
(NOT-LM-14-003)
National Library of Medicine
Requests for Applications:
Multi-Site Clinical Trials for the Pulmonary Trials Cooperative (PTC) (U01)
(RFA-HL-15-015)
National Heart, Lung, and Blood Institute
Application Receipt Date(s): October 20, 2014
Network Management Core (NEMO) for the Pulmonary Trials Cooperative (PTC) (U01)
(RFA-HL-15-016)
National Heart, Lung, and Blood Institute
Application Receipt Date(s): October 20, 2014\
Program Announcements:
Administrative Supplements for Tobacco Regulatory Research on the Role and Impact of Flavors in Cigarettes, Cigars, E-Cigarettes and Smokeless Tobacco (Admin Supp)
(PA-14-320)
Office of the Director, NIH
National Cancer Institute
National Heart, Lung, and Blood Institute
National Institute on Alcohol Abuse and Alcoholism
Eunice Kennedy Shriver National Institute of Child Health and Human Development
National Institute on Drug Abuse
Office of Disease Prevention
Application Receipt/Submission Date(s): Multiple dates, see announcement.
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

JMI Equity closed a $1 billion growth equity fund targeted at investing in software companies and services companies, it announced Wednesday.
The fund is the largest raised by JMI, a private equity firm operating out of offices in Baltimore and San Diego. The firm has raised more than $3.1 billion in committed capital since its founding in 1992. The largest fund JMI previously closed on was an $875 million fund completed in November 2010.
back to top 

When you think of tech companies, incubators or startups, South Carolina is still probably one of the last places that comes to mind. But with its new Office of Economic Engagement, which launched in the summer of last year, and a steadily growing incubator program, the University of South Carolina is working to change any misconceptions about tech startups not meshing well with the Palmetto State.
Just last week, USC’s Technology incubator held a graduation ceremony for seven companies that were ready to transition into the larger business community.
back to top 

An invasive species has been introduced into the U.S. health innovation ecosystem, with a growing danger of permanent damage to the development of specialty drugs. The relentless assault on the price of Sovaldi is becoming a threat to the 30-year political balance that has energized the biomedical revolution.
Sovaldi is the kind of medicine that the drug scolds claim to want—a true scientific advance with a near-perfect cure rate for Hepatitis C, the liver-destroying virus that infects one of every 100 Americans and some 150 million world-wide. The old critique was that pharmaceutical discovery had stalled and the industry produced only me-too drugs. Now they attack Sovaldi because its price is $84,000 a patient.
back to top 

There are approximately 1,250 business incubators in the United States, including about 120 with some life science focus, according to the National Business Incubation Association (NBIA). These programs vary greatly in their structure and services.
Often associated with universities or medical centers, life science incubators frequently offer affordable office space attached to shared wet lab facilities. Others focus on accelerating companies’ progress by offering intensive coaching, networking and support services. Some programs include seed funding up to $250,000 at a stage when few others are willing to invest.
back to top 

Digital health incubator Rock Health is investing in eight new startups and teaming up with three new corporate entities – Blue Shield of California, Deloitte and global healthcare firm Abbott.
The new startups being incubated span the healthcare spectrum, from HIPAA compliance to life sciences and biotech. Among them:
back to top 

The National Science Foundation (NSF) has awarded 36 Early Concept Grants for Exploratory Research (EAGER) to enable new technologies to better understand how complex behaviors emerge from the activity of brain circuits.
These awards will contribute to NSF’s growing portfolio of investments in support of President Obama’s BRAIN Initiative, a multi-agency research effort that seeks to accelerate the development of new neurotechnologies that promise to help researchers answer fundamental questions about how the brain works.
back to top 
In July, alongside the ÜberResearch team, we launched a Twitter competition open to PhD students. The task was to tweet how you would spend £10 million of science funding using the hashtag #uberresearchprize.
We had hundreds of tweets and the top three, as voted by our judging panel, were then invited to write a blog post, delving into their original tweet. Over the next few days we will publish the top three blog posts here on our blog, announcing the winner on Monday 25th August.
back to top 

The interest in using health IT tools as a way to improve healthcare delivery and efficiency has produced many rapidly growing healthcare companies, many of which can trace their origins within one or two years of Obamacare’s passage. These companies have reached a stage of their development to accelerate growth and that has coincided with a readiness by healthcare providers and payers to adopt or ramp up their technology.
With a nod to the top healthcare company on Inc’s 5,000 Fastest Growing Companies, molecular diagnostic companies with smartphone-enabled technology have also become more attractive as investment and acquisition targets.
back to top 

Tuesday, December 2, 2014 from 7:00 AM to 7:00 PM (EST)
The 7th Annual Maryland Stem Cell Research Symposium, hosted by the Maryland Stem Cell Research Commission and Montgomery County will be held on Tuesday, December 2, 2014 at the Silver Spring Civic Center, Silver Spring MD. The Symposium is the Maryland Stem Cell Research Fund premier event that delivers comprehensive scientific talks, poster presentations, and networking time, enabling cell therapy basic research and technologies from the lab to pre-clinical and to commercialization. With a powerful line-up of speakers and many opportunities for you to present your work in concurrent or poster presentations, the Symposium will follow the format and style of previous meetings with additional networking time and an intimate environment.
Abstract Submission:
The Maryland Stem Cell Research Fund (MSCRF) is now accepting abstract submissions for poster and oral presentations for the 7th Annual Maryland Stem Cell Research Symposium. We encourage you to take full advantage of this opportunity to share your research results with our community. Multiple abstract submissions are welcomed.
ABSTRACT SUBMISSION DEADLINE: Wednesday, September 10, 2014
Please Click Here to submit your abstract today!
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 27
American Tap Room

September 10-12
Shenzhen Convention & Exhibition Center

September 10
Shenzhen Convention & Exhibition Center

September 10-12
Washington, DC | San Francisco, CA

September 10
Germantown Innovation Center
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI) announced the appointment of four new members to its Board of Directors. Dave Lemus, Chief Executive Officer, Sigma-Tau Pharmaceuticals Inc.; Joel S. Marcus, Chairman, Chief Executive Officer, and Founder, Alexandria Real Estate Equities, Inc. (Alexandria Venture Investments); and Beth Meagher, Principal, Deloitte Consulting LLP, have joined the BHI Board of Directors, effective August 7, 2014, based upon their organizations’ newly committed support of BHI. Charles “Chuck” Morton now represents Venable LLP on the Board, as another new Board member, while Mike Baader, formerly with Venable, will continue to hold a seat on the Board, but now as a representative of Greenspring Associates. Cynthia L. Collins, CEO, Clarient, who had served on the BHI Board since early 2013, is stepping down from her Board position after relocating to the West Coast
“The expansion of and continued diversity within the BHI Board, based upon new key industry and service provider representatives who are all deeply involved in life sciences, is a testament to the positive impact BHI has made on the Central Maryland biohealth community to date,” said Richard Bendis, BHI President & CEO.
“We look forward to the contributions from the new Board members representing these organizations. We’re also pleased to welcome a new Venable representative to the Board and that Mike Baader will continue to add value to the organization now that he is on the Greenspring Associates team,” said Douglas Liu, Chair, BHI Board of Directors, and Senior Vice President of Global Operations for Qiagen. “And, on behalf of the BHI staff and Board, I would also like to offer gratitude to Cynthia Collins for her service and contributions to the BHI Board over the last year and half.”
back to top 

BioHealth Innovation, Inc. (BHI), a Montgomery County innovation intermediary which translates market-relevant research into commercial success by bringing together among other things management, funding, and markets, is seeking an experienced life science professional with entrepreneurial experience to serve as an Entrepreneur-in-Residence (EIR).
The EIR will lead in the evaluation of early-stage technologies and corporations with a priority focus on those associated with Funding Partners and those that best fit with the strategic direction as set by BHI’s CEO and Director of EIR Programs. The EIR will perform any reasonable task which will forward the goals of BHI. Further specifics and responsibilities include:
back to top 
The 2014 International Biotech Summit Committee invite BioHealth Innovation (BHI) and Montgomery County to attend the three co-located international conferences that will be held on Sep 9 -12 in Shenzhen, China:
- 2014 International Biotech Summit (September 10, 2014);
- The 9th International Conference on Genomics (ICG-9) (September 11th-12th, 2014);
- Shenzhen International Biotech & Health Industry Expo 2014 (SIBHIE, September 10th-12th, 2014).
Ms. Lily Qi, Director of Special Projects for Montgomery County Executive Office who was involved in establishing BHI and a native of Shanghai, is going to speak on behalf of the County and BHI for the forum, “Innovation in Policy and Regulation for Advancing Healthcare Industry.” BHI and the County are also invited as an exhibitor for the SIBHIE Expo.
back to top 
Montgomery County Department of Economic Development (DED) announces the addition of Valerie Fremont as Senior Business Development Specialist for the County’s life sciences and health IT sectors. Fremont brings 13 years of industry experience to the County along with experience in the international, national and regional biotech community.
”We are happy to welcome Valerie to the department and fortunate to be able to call upon her vast experience in the life sciences arena,” said DED Director Steve Silverman. “Not only does she bring a wealth of knowledge of two of our most important sectors—life sciences and health IT—but she also is already well known in that community. The County and our companies in those sectors will all benefit mutually from those important connections.”
back to top 

August 20th, 3-5 PM
MdBio Foundation welcomes you to an open house on the mobile laboratory MdBioLab. Tour the lab, meet the team, or just eat your ice cream – doors open 3 – 5 PM.
KICK OFF THE SCHOOL YEAR WITH AN ICE CREAM CONE AND TOUR OF MDBIOLAB.
MdBioLab will be parked at Howard Community College, 10901 Little Patuxent Pkwy, Columbia, MD 21044, Lot F – adjacent to Little Patuxent Parkway
back to top 

Wrap up a great summer with one last BioBuzz networking event on August 27th from 5:00 – 7:30 p.m. at American Tap Room in Rockville, MD. This location is a short walk from the Metro located in the Rockville Town Center so there are no excuses not to come.
New this month, join us for Table Talks at BioBuzz.
This month’s featured Table Topics will be on Project Management & Navigating your Life Science Careers.
Life Science Career Talks with;
Shira Harrington, President of Purposeful Hire
Multiple corporate recruiters from local Biotech companies
Project Management discussions with;
Tariq Allana, Project Manager at GlycoMimetics
Allen Bolden, Experienced GMP Manufacturing Project Manager
back to top 

Sigma-Tau Pharmaceuticals (Sigma-Tau) has signed an exclusive US license agreement with Ireland-based Crosscare, to market and distribute Colief Infant Drops for the treatment of colic.
As part of the deal, Sigma-Tau will be responsible for the sales, marketing and distribution of Colief in the US.
Sigma-Tau has planned a re-launch of Colief at healthcare professionals as well as consumers in August 2014.
back to top 

The Rockville Science Center has found a temporary office home in the midst of an ongoing search for a permanent facility.
The science center’s administrative office, previously at the VisArts at Rockville center, moved this month to the Johns Hopkins University’s campus in Rockville.
back to top 

A unique summer internship program established by the Universities at Shady Grove (USG) and funded in part by the University of Maryland, Baltimore (UMB), has enabled 12 students to work across disciplines in a community setting.
Based at the Rockville, Md., campus where UMB offers a growing array of programs, the eight-week internship was conducted within the Montgomery County Department of Health and Human Services (DHHS). Four graduate and eight undergraduate students experienced interdisciplinary teamwork by visiting several service agencies throughout Montgomery County.
back to top 

Johns Hopkins University’s FastForward business accelerator is so popular the school is expanding it to a second location.
FastForward, which is part of Hopkins’ Whiting School of Engineering, launched last year and is already at capacity with a total of 33 startups and projects. Administrators plan to expand the program in January to accommodate the high demand among students and faculty.
back to top 

The BioMaryland Center is partnering with Maryland’s Department of Health and Mental Hygiene (DHMH) and the Center for Medical Technology Policy (CMTP) to incorporate improved health care quality and cost reduction criteria in the selection process for the BioMaryland Center’s annual Biotechnology Awards program. $1M will be awarded to projects, $50,000-200,000 each, advancing technologies toward commercialization–with preference given to projects which improve patient outcomes and reduce costs.
October 1st is the deadline to apply for the next round of Biotechnology Development Awards. More information regarding the program is available online.
How to Apply: Two application forms (the Non-confidential Applicant and Project Summary form and the Confidential Application Details form) and a business plan must be submitted electronically by 5 p.m. EST on October 1, 2014.
back to top 

The Maryland Venture Fund (MVF), the equity investment arm of the Maryland Department of Business and Economic Development (DBED), has made follow-on investments totaling $700,000 in two of its portfolio companies. KoolSpan, developer of a suite of patented, hardware-based mobile security encryption solutions, received $400,000. BrainScope, which is pioneering sophisticated neurotechnology to quickly assess traumatic brain injury at the initial point of care, received $300,000. Both companies are located in Bethesda. The investments were made with funds raised by InvestMaryland, Governor Martin O’Malley’s key initiative designed to spur growth and development of small, high-tech companies in Maryland.
“KoolSpan and BrainScope have both made tremendous progress since the Maryland Venture Fund first invested in them, and we are excited to make these follow-on investments,” said Governor O’Malley. “In Maryland we are committed to supporting the growth of future leaders in industries that keep us safer, healthier and better connected than ever before. KoolSpan and BrainScope are perfect examples of the innovative, high-tech companies that set Maryland’s economy apart and provide the family-sustaining jobs of today and tomorrow.”
back to top 

When four professors of the Indian Institute of Science (IISc), Bangalore, decided to start Strand Life Sciences in 2000 to aid clinical research through genomic testing using DNA sequencing, the initiative hardly attracted attention. UTI Ventures pumped in $1.3 million a year later and, in 2002, WestBridge Capital Partners invested $1.9 million, but that did not create ripples either. However, things changed dramatically in 2013, when Strand got $10 million from San Francisco-based financial firm Burrill & Co.
It used the capital to roll out personalised genetic test services in collaboration with hospital chains and clinics across India, including Max Hospitals, Mazumdar-Shaw Cancer Center, St. John’s Medical College Hospital, Kidwai Memorial Institute of Oncology and HealthCare Global Enterprises.
back to top 

GlaxoSmithKline, better known as GSK, has launched a new web effort aimed at providing both information and inspiration for people with chronic obstructive pulmonary disease, in what the company says is arguably the most engaging platform in the marketplace.
The rollout of the new COPD.com goes beyond what was previously offered, certainly by GSK but possibly many others, according to Darielle Ruderman, senior director of repository consumer marketing for the North Carolina-based company.
back to top 

The answers Bert Vogelstein needed and feared were in the blood sample.
Vogelstein is among the most highly cited scientists in the world. He was described, in the 1980s, as having broken into “the cockpit of cancer” after he and coworkers at Johns Hopkins University showed for the first time exactly how a series of DNA mutations, adding up silently over decades, turn cells cancerous. Damaged DNA, he helped prove, is the cause of cancer.
Now imagine you could see these mutations—see cancer itself—in a vial of blood. Nearly every type of cancer sheds DNA into the bloodstream, and Vogelstein’s laboratory at Johns Hopkins has developed a technique, called a “liquid biopsy,” that can find the telltale genetic material.
back to top 

DC I-Corps, a new, NSF-supported program designed to foster, grow and nurture an innovation ecosystem in the Mid-Atlantic Region, is now accepting applications for its fall cohort, beginning on October 9. Applications will be accepted on a rolling basis up until that date. Apply here.
Open to research teams and technology entrepreneurs from universities, federal laboratories, agencies and the general community, the free program guides researchers in exploring the commercial potential of their inventions.
Through DC I-Corps, you will:
- Significantly improve your chances for SBIR and other grant funding, as well as early stage venture investment;
- Work closely with six or more real-world advisors that have startup, venture capital, and technology commercialization experience over a six-week period; and
- Come to a clear go or no-go decision regarding the commercial potential of your technology.
The program is geared towards innovations coming from engineering fields, medical/health/life sciences, and physical and computer sciences. DC I-Corps builds upon the successful National Science Foundation (NSF) I-Corps program.
The program is jointly offered by the University of Maryland, George Washington University, Virginia Tech, and Johns Hopkins University. For more information and to apply, technology researchers and entrepreneurs are encouraged to visit www.dcicorps.org.
back to top 

Are you a company in the fields of cloud computing, network security, interoperability, chemical and biological defense, infectious disease or vaccines? Then you’ll want to attend the Maryland Department of Business and Economic Development’s Defense Labs Tech Transfer event which includes panel discussions and a tech transfer showcase. Hear from Federal Labs including NSA, DISA, RDECOM, Edgewood Chemical Biological Center (ECBC), and USAMRIID, see demonstrations, speak with inventors, discuss available patents, licenses, collaborations and commercialization of new technologies to add to your portfolio.
Featuring:
Tech Transfer Showcase 9:00 a.m. – 4:00 p.m. – Visit the exhibit floor and see the latest technologies being developed by NSA, DISA, RDECOM, USAMRIID, and the Edgewood Chemical Biological Center.
back to top 

Innovative technologies and methodologies fuel progress in biomedical and behavioral research and represent an increasingly important area of the economy. The Small Business Innovation Research (SBIR) program provides support for research and development (R&D) of new or improved technologies and methodologies that have the potential to succeed as commercial products.
The purpose of this notice is to (1) announce the issuance of the Solicitation of the National Institutes of Health and the Centers for Disease Control and Prevention for Small Business Innovation Research Contract Proposals (PHS 2015-1) with a receipt date of November 5, 2014, 4:30 PM ET; and (2) inform the public about the opportunities that the SBIR program offers to small business concerns as well as to scientists at research institutions.
The SBIR legislation requires the Public Health Service (PHS), Department of Health and Human Services, and certain other Federal agencies to reserve 2.8 percent (for FY 2014) of their extramural research or R&D budgets for an SBIR program. (The NIH SBIR set-aside requirement for FY 2014 is $663 million.)
back to top 

The National Institutes of Health is challenging science innovators to compete for prizes totaling up to $500,000, by developing new ways to track the health status of a single cell in complex tissue over time. The NIH Follow that Cell Challenge seeks tools that would, for example, monitor a cell in the process of becoming cancerous, detect changes due to a disease-causing virus, or track how a cell responds to treatment.
“Advances in cellular analysis promise earlier diagnosis and improved therapies for diseases, from cancer to Alzheimer’s,” said James Anderson, M.D., Ph.D., director of NIH’s Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI). “These prizes will also help to stimulate new businesses and economic growth in our biomedical communities.”
back to top 

For the third year in a row, Johns Hopkins has been recognized as one of the nation’s best college campuses for dining.
Hopkins ranks second on the list of the “Best Colleges for Food in America” compiled by the website The Daily Meal, up from a No. 42 ranking a year ago. The site praised JHU’s commitment to sustainability, the diversity of food offerings, and its special programming, including on-campus cooking classes and food and wine pairings for seniors.
back to top 

For an incubator set on changing perceptions of where startups need to be to succeed in regulated sectors such as healthcare, education and energy, the decision to set up 1776 in Washington D.C. was a smart move. Mentors abound in a city where lawmakers, think tanks, regulators and Fortune 500 companies come to do business. In a phone interview, co-founder Donna Harris talked to MedCity News about its approach to finding and working with startups.
Harris co-founded 1776 in 2012 with Evan Burfield. Harris had previously worked at Startup America Partnership (which later changed its name to UP America) as managing director, and before that she was Vice Chair of Interpoint. Burfield founded netDecide and Synteractive.
back to top 

Luminal, a Frederick and D.C.-based cloud management startup, on Thursday announced a $10 million Series B round led by New Enterprise Associates.
Joining NEA are prior investors Core Capital Partners and the Maryland Venture Fund. The latest financing brings Luminal’s total funding to $13.8 million.
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- NIH Offers Commercialization Assistance Program to Phase II SBIR and STTR Awardees
- Revised Policy: Descriptions on the Use of Individual Development Plans (IDPs) for Graduate Students and Postdoctoral Researchers Required in Annual Progress Reports beginning October 1, 2014
- Notice of NICHD’s Participation in PA-14-016 “Ruth L. Kirschstein National Research Service Award (NRSA) Short-Term Institutional Research Training Grant (Parent T35)
- (NOT-HD-14-026)
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

The rich are getting richer when it comes to publicly traded biotechnology companies, judging by their ballooning market capitalization. It’s a product of the current Wall Street surge—some call it a bubble—that continued in the first half of 2013, with 52 companies going public and the overall market closing at record highs.
Following is a list of 25 biotechnology companies, ranked by their market cap as of July 24 as furnished by the exchanges on which they trade their shares, or by other publicly available sources, such as any of several free stock information websites. Figures of non-U.S. companies were converted to U.S. dollars from various currencies.
back to top 

Monday, September 22, 2014
NHLBI’s Office of Translational Alliances and Coordination (OTAC) hosts this semi-annual Regional Innovation Conference to connect small businesses, angel investors, venture capitalists, strategic partners, and business leaders from the biotech, medical device, and pharmaceutical industries. Attendees will see featured presentations from NHLBI-funded companies, and learn from staff about recent changes in the Federal SBIR/STTR program, and other funding opportunities and cost-free resources available to small businesses.
Previous conferences have been held in Boston, MA, San Francisco, CA, San Diego, CA, and Rockville, MD.
Highlights/ Features:
- Company presentations
- Hear about innovative technologies for heart, lung, blood and sleep disorder and disease indications from companies who have received (non-dilutive) SBIR/STTR funding
- How to market your innovation
- Our expert panel of investors, legal advisors, and successful entrepreneurs will talk about the highs and lows of early-stage life science investment
- Networking
- Ample opportunities to network with potential partners, investors, and NHLBI staff
back to top 

Today, the American Heart Association has launched the Chicago Open Innovation Challenge, a crowdfunding competition to uncover new innovative digital health tools to prevent or manage heart disease and stroke. The top three finalists will receive grants from the American Heart Association totaling $25,000 and a chance to present at the Heart Innovation Forum in Chicago on November 14, 2014. Each award winner will be featured on healthcare crowdfunding platform MedStartr.
Who Can Apply? Early-stage healthcare technology and life sciences companies with novel ideas that seek to help patients, providers or medical facilities meet the American Heart Association’s 2020 Impact Goal—to improve the cardiovascular health of all Americans by 20 percent while reducing death from cardiovascular diseases and stroke by 20 percent by the year 2020—are encouraged to apply.
back to top 

Traditional Chinese Medicine (TCM) has been used for thousands of years to prevent and treat disease and promote wellness. But, in many western countries, there had been historic resistance to its use because it “was not supported by rigorous scientific study.”
That’s not true today.
back to top 

Digital health has a pretty erratic grade point average, or so says Dana Mead, a parter at venture firm Kleiner Perkins, Caufield & Byers. He outlined and graded what he called the five most important elements of the digital health ecosystem at Qualcomm Life‘s Connect2014 conference this week in San Diego, and this is how he slices it:
1. Access to Capital – A
Looking at data over the last quarter, Mead said that about $700 million in funding has been raised in digital health. That’s more than medical device startups raise, about two-thirds of what pharma and biotech raise and about a third of what software/IT companies raised in the same time period. So, not a bad showing for digital health, Mead said.
back to top 

The organizers of the 2nd Annual Symposium for Pediatric Surgical Innovation and Competition judges are seeking proposals from inventors in medical institutions, private practices, device companies, and academic researchers who have medical device concepts or ideas for use with pediatric patients.
Proposals should address a significant, yet unmet need within the pediatric population with a device idea that lends itself to commercialization. Following the competition process, two prizes will be given, each for up to $50,000.
Please follow this link http://www.pediatric-surgery-symposium.org/prizes/ to review the submission guidelines and to submit your application online by September 22, 2014 at 17:00 EST.
back to top 

The mobile revolution was one of the most transformative events in IT. It set IT in a tailspin trying to secure so many different device types connecting to the network and moving outside of the organization.
After initial resistance, IT realized that by supporting mobility they were supporting user productivity. And thus an established industry of mobile device management solutions was born, allowing IT to create policies and strike a balance between data security and mobility.
back to top 

Much of the discussion on big data has focused on claims information from insurers and EHRS from providers, but a collaborative effort underway at Stanford Medical School with SAP is hoping to tap into a different set – genomic data.
The possible benefits of sharing genetic data on a wide scale have great potential for both global population health and for drug makers alike, said Dr. Carlos Bustamante, who heads the Department of Genetics at the Stanford School of Medicine. Benefits range from possibly learning why certain drugs never make it out of clinical trials because of what population they are tested on to a more inclusive global snapshot at differing populations and what drives their health spending, among others.
back to top 

The annual global confidence survey from the National Venture Capital Association produced an unusual apparent contradiction.
Investors around the world think the U.S. is the best place to back startups but confidence in our country’s government among domestic VCs is dropping.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

August 20
Howard Community College

August 27
American Tap Room

September 10-12
Shenzhen Convention & Exhibition Center

September 10
Shenzhen Convention & Exhibition Center

September 10-12
Washington, DC | San Francisco, CA
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
| | | | | | | | | | | | | | |