|
|
|

Immunotherapy is at the forefront of cancer care; however, most cancers evolve the ability to dodge the body’s defenses.
Maryland startup BeneVir is developing immunotherapy viruses that rid the body of two types of tumor cells – those that cause cancer and the ones that make it recurrent.
back to top 

British drugmaker AstraZeneca plans to spend $200 million over the next three years, expanding its manufacturing facility in Frederick, Md., and hiring an additional 300 workers at the site, executives said.
The decision further cements Gaithersburg-based MedImmune, an AstraZeneca company, as the crown jewel of Maryland’s life sciences industry.
back to top 

An experimental vaccine to prevent Ebola virus disease was well-tolerated and produced immune system responses in all 20 healthy adults who received it in a Phase 1 clinical trial conducted by researchers from the National Institutes of Health. The candidate vaccine, which was co-developed by the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and GlaxoSmithKline (GSK), was tested at the NIH Clinical Center in Bethesda, Maryland. The interim results are reported online in advance of print in the New England Journal of Medicine.
back to top 

The stampede is back on among venture capital firms to raise new money and close more funds, after years of standing pat following the recession and years of sluggish recovery.
back to top 

That next blockbuster drug? It all begins with a hypothesis: GlaxoSmithKline just announced the winners of its second Discovery Fast Track Challenge – a competition that teams up American and European academia with GSK researchers to speed up their search for new therapeutics.
back to top 

The Washington Business Journal interviewed Leslie Ford Weber, JHU’s director of the Montgomery County Campus and of government and community affairs for Montgomery County. The feature ran as an Executive Profile on Nov. 14, 2014. It was written by Vandana Sinha, an assistant managing editor at the Washington Business Journal. The photo was taken by Joanne S. Lawton.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
Notices:
- NIH Implementation of the US Government Policy on Institutional Oversight of Life Sciences Dual Use Research of Concern
- Publication of Notice of Proposed Rulemaking for Clinical Trials Registration and Results Submission under FDAAA
- NIH Request for Public Comments on the Draft NIH Policy on Dissemination of NIH-Funded Clinical Trial Information
- Request for Information (RFI): Inviting Comments and Suggestions on the Reagent-Related Barriers to Reproducible Research
- Reminder: Annual Reports to the Office of Laboratory Animal Welfare due January 31, 2015
- Notice of Intent to Publish a Funding Opportunity Announcement for Sudden Death in the Young: Population-Based Studies (U01)
Program Announcements
- Systems Science and Health in the Behavioral and Social Sciences (R21)
- (PAR-15-047)
- Office of Behavioral and Social Science Research
- National Cancer Institute
- National Institute on Aging
- National Institute on Alcohol Abuse and Alcoholism
- National Institute of Biomedical Imaging and Bioengineering
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Dental and Craniofacial Research
- National Institute of Environmental Health Sciences
- National Institute of Mental Health
- National Institute of Nursing Research
- Office of Disease Prevention
- Application Receipt/Submission Date(s): Multiple dates, see announcement.
- Systems Science and Health in the Behavioral and Social Sciences (R01)
- (PAR-15-048)
- Office of Behavioral and Social Science Research
- National Cancer Institute
- National Institute on Aging
- National Institute on Alcohol Abuse and Alcoholism
- National Institute of Biomedical Imaging and Bioengineering
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute of Dental and Craniofacial Research
- National Institute of Environmental Health Sciences
- National Institute of Mental Health
- National Institute of Nursing Research
- Office of Disease Prevention
- Application Receipt/Submission Date(s): Multiple dates, see announcement.
back to top 

In 2015, hospitals will – and should – make more advanced use of “third platform” technologies based on mobile tools, social channels, data analytics and the cloud, according to a recent report from IDC Health Insights.
With healthcare costs unsustainable, but these new technologies now ubiquitous, IDC officials say hospital CIOs will increasingly be turning to new tools – especially as consumers expect healthcare to be as responsive to their wants and needs as other industries.
back to top 

Angel investors are often rich individuals who provide startups with capital for their start-up costs. The term comes from Broadway, where it was originally used to describe the wealthy individuals who provided money for theatrical productions.
back to top 

Myron M. Levine, MD, DTPH, director of the University of Maryland School of Medicine Center for Vaccine Development (CVD), and Dean E. Albert Reece, MD, PhD, MBA, announced today the start of a clinical trial in Baltimore to evaluate different dosage levels of a promising experimental Ebola vaccine developed by the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and GlaxoSmithKline (GSK
back to top 

The Federal Laboratory Consortium for Technology Transfer (FLC), a nationwide network of federal laboratories that cultivates best-practice strategies for advancing technology transfer (T2) from the laboratory to the marketplace, today announced the launch of FLCBusiness, an interactive business resource tool.
back to top 

Lacrosse sticks, construction models and surgical tools — these are all things Baltimore companies are making with the help of 3-D printing.
Three-dimensional printing was invented decades ago but has really taken off in the last few years. Printers are more affordable (you can get one for your desktop for the price of a MacBook Pro). And printing material has advanced significantly, to include more durable plastics, metal and more.
back to top 

Every once and a while we get a clear example of the gulf between those battling over important public policy issues and can understand why the public and policy makers are confused by resulting charges and counter charges. Last week was a good illustration.
back to top 
David Chalker, 50, has excruciating pain in his hip. He’s an Army veteran and because of the pain, he had to leave his job as a machinist, which left him in a great deal of debt and unable to pay for health insurance. He, his wife, and his three daughters needed to move in with his in-laws as a result.
back to top 

The color of your urine could be telling you something about your health condition. Yes, your standard yellow is where you want to be, but the different shades of the rainbow make an appearance on occasion.
back to top 

Any small business or venture capital company interested in Small Business Innovation Research (SBIR) or Small Business Technology Transfer (STTR) funding opportunities should pay close attention to the Small Business Administration’s (SBA) recent request for public comments, by January 6, 2015, on data rights and Phase III funding, and a recent Government Accountability Office (GAO) report identifying the National Institutes of Health (NIH) and Department of Energy’s Advanced Research Projects Agency-Energy (ARPA-E) as the two agencies presently accepting applications from majority-owned portfolio companies.
back to top 

Nestled in a quiet industrial park in Redmond, Washington, not too far from the Microsoft headquarters, is a small biotech start-up with both an interesting technology they are bringing to market, as well as a capital partner that suggests some ways in which global biotech research, venture capital and commercialization are going to change.
back to top 

Small businesses are a major driver of high-technology innovation and economic growth in the United States, generating significant employment, new markets, and high-growth industries.1 In this era of globalization, optimizing the ability of small businesses to develop and commercialize new products is essential for U.S. competitiveness and national security. Developing better incentives to spur innovative ideas, technologies, and products—and ultimately to bring them to market—is thus a central policy challenge.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

December 5
Baltimore

December 8
Johns Hopkins Medical Campus

December 10
Germantown Innovation Center

December 10
Johns Hopkins University Montgomery County Campus

May 7- 8
Bethesda Mariott
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BeneVir Biopharm, Inc., a biotechnology company developing a pipeline of cancer immunotherapies, today announced it closed a Series A investment round with Pansend, LLC, an indirect wholly owned subsidiary of HC2 Holdings, Inc. (OTCQB: HCHC).
BeneVir’s core technology was licensed from New York University (NYU) and originally developed by Ian J. Mohr, Ph.D., NYU Langone Medical Center.
back to top 

The Life Sciences Impact Grant Program was created in 2014 to provide financial assistance to life science employers who retain jobs and to stimulate the organic growth of life sciences in Montgomery County.
The Department of Economic Development will award grants in the amount of between $5,000 and $25,000 to 5-7 life sciences companies, enabling them to advance a business development/product development goal.
back to top 

British drugmaker AstraZeneca is doubling down on Maryland, spending $200 million to expand its Frederick manufacturing facility a year after shifting hundreds of out-of-state jobs to Montgomery County.
back to top 

Drug giant AstraZeneca will expand its biologics manufacturing center in Frederick and add hundreds of jobs to its operations there.
The drug giant will spend more than $200 million to increase production capacity. MedImmune, AstraZeneca’s biologics research and development arm, has more than 120 drugs in its research pipeline, including more than 30 in clinical development. AstraZeneca says the expansion will support its research.
back to top 

Local companies, along with County Executive Leggett, are exploring incredible international business opportunities in the Indian market as part of the County’s business mission to India November 13 through 22. Read the press release to learn more.
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association for life science and technology, today announced that it will honor former Lockheed Martin Corporation Chairman and CEO Norman Augustine, MedImmune, Inc. Founder Dr. Wayne Hockmeyer and University System of Maryland Chancellor William Kirwan with its 2015 Lifetime Achievement Awards. The awards will be presented during TCM’s Lifetime Achievement Gala, February 19, 2015.
back to top 

Baltimore biotech firm Noxilizer Inc. is expanding its footprint at the University of Maryland BioPark to meet growing demand from clients.
Noxilizer developed a medical sterilization system that uses nitrogen dioxide, a faster alternative to traditional methods. The company added about 5,000 square feet to its sterilization space to accommodate a growing interest in its contract sterilization services. The company’s sterilization lab now takes up about 16,00 square feet — the entire basement at 801 W. Baltimore Street.
back to top 

Protagen AG announced that it has entered into a long-term collaboration agreement with QIAGEN targeting the development of novel protein-based companion diagnostics for autoimmune disorders. Under the terms of the agreement, QIAGEN will gain access to the proprietary SeroTag® technology platform of Protagen, which enables the discovery and validation of novel protein-based marker panels. Such markers hold great promise for the development into companion diagnostics to guide treatment decisions in various autoimmune disorders. Financial details of the collaboration were not disclosed.
back to top 

Two University System of Maryland schools are turning investor.
University of Maryland, Baltimore, and University of Maryland, College Park plan to invest up to $500,000 each in Maryland-based startups that use technology licensed from the universities.
back to top 

What do beekeepers, marching band members, engineers and choir singers all have in common? They all raised money for various projects through Launch UMD, a new crowdfunding platform at the University of Maryland, College Park.
(Screenshot courtesy of launch.umd.edu)
back to top 

Britain’s GlaxoSmithKline (GSK.L) has asked its shareholders to vote at a meeting on Dec. 18 on its proposed major deal with Switzerland’s Novartis (NOVN.VX), which will see the two pharmaceutical group trade more than $20 billion of assets.
back to top 

Myron M. Levine, MD, DTPH, director of the University of Maryland School of Medicine Center for Vaccine Development (CVD), and Dean E. Albert Reece, MD, PhD, MBA, announced today the start of a clinical trial in Baltimore to evaluate different dosage levels of a promising experimental Ebola vaccine developed by the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and GlaxoSmithKline (GSK
back to top 

There are presently massive shifts occurring in the competitive global landscape of health, and particularly in the life sciences. As we approach 2015, it is imperative that leaders in the health space understand the trends and shifts happening around them, not only in the US, but also in international markets, cities and service lines.
back to top 

Surgery is an art form for Johns Hopkins surgeon Dr. Amir Dorafshar, who on a November morning spent four hours smoothing the point out of a child’s skull.
The procedure, needed to allow the 18-month old’s brain to grow properly and avoid developmental disorders, requires taking apart the skull in pieces, then putting it back together.
back to top 

Few businesses owned by venture capital firms have been awarded Small Business Innovation Research awards since this program was opened to them two years ago.
Through the SBIR program, 11 federal agencies spend at least 2.8 percent of their outside research budgets with small businesses. Only two of these agencies — the National Institutes of Health and the Advanced Research Projects Agency-Energy — have allowed VC-owned businesses to compete for SBIR awards, according to a new Government Accountability Office study.
back to top 

A Johns Hopkins biomedical engineering student team has placed second in the undergraduate division of the Collegiate Inventors Competition for its AccuSpine probe, marking the third consecutive year that a Johns Hopkins team has been awarded a top prize in this challenge.
back to top 
The president of the University of Maryland University College, rejecting a recommendation from an outside committee, has decided he won’t ask the state to let the university convert to a private nonprofit institution or break away from the University System of Maryland.
back to top 

Wednesday, December 10, 2014 from 6:00 PM to 8:00 PM (EST), Rockville, Maryland
Climbing the Regulatory Summit: Insights into developing the Best Regulatory Pathway for your Venture and Methods of Designing an Efficient and Productive Clinical Trial
An absolutely essential exercise in any healthcare or life science start-up is to determine the optimal regulatory pathway and the most efficient clinical trial design. Come and hear the experts before you go spending those scarce resources!
back to top 

Are you an established public company looking to impress investors with your company’s latest developments, or a late-stage private company hoping to make the valuable connections needed to take your product to the next phase? Nominate your company to be the Buzz of BIO at the 2015 CEO & Investor Conference!
Ten biotechs will be nominated in each of two categories, “Most Distinguished Public Company” and “Most Promising Private Company.” Only 20 nominations will be accepted in total, and must be submitted by 5:00pm ET, November 21, 2014. All nominations are subject to review.
back to top 

The angel investor market in Q1,2 2014 showed signs that the five year moderate growth has continued in the first half of 2014. Total investments in Q1,2 2014 were $10.1 billion, an increase of 4.1% over Q1,2 2013, according to the Center for Venture Research at the University of New Hampshire. A total of 30,270 entrepreneurial ventures received angel funding in Q1,2 2014, a 5.9% increase from Q1,2 2013, and the number of active investors in Q1,2 2014 was 143,140 individuals, an increase of 6.1% from Q1,2 2013. The increase in total dollars and the larger increase in total investments (deals) resulted in a deal size of $332,120 in Q1,2 2014, a decline from the deal size in Q1,2 2013 of $337,850. These data indicate that angels remain major players in this investment class and at valuations similar to Q1,2 2013. The market exhibited a sustained growth pattern over a five year period and the angel market has now recovered from the correction in 2008.
back to top 

Big pharma companies are making greater efforts to improve access to medicines in the developing world, but corruption and intellectual property (IP) issues are areas of concern, says a new report.
The biannual Access To Medicines Index (ATMI) – which is funded by the Bill & Melinda Gates Foundation and the UK and Dutch governments – puts GlaxoSmithKline at the top of its rankings in 2014 for the fourth time, followed by Novo Nordisk and Johnson & Johnson.
back to top 

As part of ongoing research into national healthcare spending, the Deloitte Center For Health Solutions recently published their findings based on health data from 2012. According to the new report, there’s an additional amount of healthcare consumer spending that isn’t included in the federal calculations (often referred to as the National Healthcare Expenditure or just NHE). The new Deloitte calculations represent out‒of‒pocket expenses by consumers and amount to an additional $672 billion for 2012. By Deloitte’s accounting, this additional amount puts the NHE for 2012 at $3.46 trillion.
back to top 
As the sound of pile driver at the Edward St. John’s Learning and Teaching Center construction site boomed across Campus Drive, officials broke ground Friday morning on another project: a bioengineering building aimed at forwarding research in a relatively new field.
back to top 
Digital technologies such as electronic medical records, mobile devices, and analytics offer the potential to transform health care. Whether it’s a patient using her smartphone to better manage her diabetes, a provider monitoring a patient for arrhythmia remotely, or an electronic health-record system alerting a clinician of a potential drug allergy, digital technologies can create meaningful value for patients and practitioners alike. Yet there are significant barriers to the development and adoption of such technologies that academic medical centers are uniquely positioned to overcome.
back to top 

ATCC, the premier global biological materials resource and standards organization, is pleased to announce that Ralph Koch has joined ATCC as the Senior Vice President of Finance and Administration/Chief Financial Officer and Dr. James Kramer has joined as the Vice President of Operations.
ATCC has a reputation for delivering high quality biomaterials and services to support scientific research and breakthroughs that improve the health of global populations.
back to top 

That was the theme when medical thought leaders converged on New York City recently for the Faster Cures Center of the Milken Institute’s “Partnering for Cures” conference where Fox News’ own Dr. Manny Alvarez was a panel moderator.
back to top 

Unlock your molecule’s potential with the help of EMD Millipore’s Emerging Biotech Grant Program
At EMD Millipore, it’s our goal to help advance lifesaving drugs to market. We understand the challenges that emerging biotech companies face in their quest to quickly push the next generation of drugs to market. We want to help you succeed.
back to top 

In perhaps yet another sign of the expanding role of retail healthcare, CVS Health said it is opening a new Digital Innovation Hub on Boston that will serve as a central hub for the Rhode Island-based pharmacy’s digital health team.
back to top 

The Department of Health and Human Services has expanded its entrepreneurs-in-residence program with new partners who will work with agency employees on various projects over a 12-month period.
back to top 

Now through December 1st, 2014, Freudenberg will be hosting a contest in the area of smart surface technology for medical devices (preferably silicone based). Share your new product or business idea for the opportunity to launch a successful and innovative startup business in cooperation with Freudenberg.
back to top 

Ten years ago, scientists discovered that some people are naturally missing working copies of a gene known as PCSK9. The consequences of the mutation were extraordinary. These people, including a Texas fitness instructor, a woman from Zimbabwe, and a 49-year-old Frenchman, had almost no bad cholesterol in their blood. Otherwise, they were perfectly normal.
back to top 

In the “Mystery of Capital,” Peruvian economist Hernando de Soto famously writes about the need to convert assets into capital for creation of social and economic value in developing countries and economies in transition, noting: “Any asset whose economic and social aspects are not fixed in a formal property system is extremely hard to move in the market.” While de Soto is describing the need to legalize informal property systems, this is equally true with respect to BRICS and other countries seeking to unlock capital resources for R&D intensive start-ups, also known as Micro, Small and Medium Enterprises (MSMEs).
back to top 
The US healthcare industry is undergoing a major transformation as healthcare reform encourages consumers to play a far more active decision-making role. Yet despite this traditionally business-to-business industry moving quickly to a business-to-consumer model, companies have been slow to join the digital movement. Unlike successful B2C companies in other industries—which offer mobile solutions, provide personalized product recommendations, and empower customer-service agents with a 360-degree view of the customer—most healthcare providers and payors are lagging, as are pharmaceutical companies and medical-device manufacturers.
back to top 

More than half of all healthcare practitioners, or 57 percent, said that on “most days” they feel more attached to computers than their patients, according to a recent survey conducted at the Integrative Health and Medicine conference.
back to top 

Patients and doctors often don’t know if surgery to remove cancerous tissue was successful until scans are performed months later. A new kind of nanoparticle could show patients if they’re in the clear much earlier.
The nanoparticles—dubbed nanoflares—attach themselves to individual cancer cells in a blood sample and then glow, allowing cancerous cells to be detected and sorted with the help of a laser.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

December 5
Baltimore

December 8
Johns Hopkins Medical Campus

December 10
Germantown Innovation Center

December 10
Johns Hopkins University Montgomery County Campus

May 7- 8
Bethesda Mariott
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

QIAGEN N.V. (Nasdaq: QGEN) today announced it has entered into a master collaboration agreement with the Swiss pharmaceutical company Novartis AG (NYSE: NVS) to enable the development and commercialization of companion diagnostics to be paired with existing Novartis pharmaceutical products as well as compounds in its development pipeline.
The non-exclusive agreement with Novartis creates a framework for collaborations that would include developing QIAGEN companion diagnostics to guide treatment decisions for Novartis pharmaceutical products. The scope of the collaboration can cover all QIAGEN platforms, indications or biomarkers. Financial terms of the agreement were not disclosed.
back to top 

LabCentral and Roche announced an agreement in which Roche would provide technology and financial support to LabCentral, a first-of-its-kind shared laboratory space designed as a launchpad for high-potential life-sciences and biotech startups.
back to top 

WellDoc®, a leading digital health care behavioral science and technology company has launched a multi-stage collaboration effort with Samsung Electronics Co. Ltd, to improve the lives of those living with type 2 diabetes and explore next generation diabetes devices and product offerings.
View gallery . The parties share a vision for leveraging technology to empower patient self-management and provider clinical decision-making. They each have commercialized unique and powerful health care platforms that when combined, can deliver unparalleled support to patients with diabetes.
back to top 

Startup Maryland (http://www.startupmd.org) today announced the Great two top Finalists from the 2014 Pitch Across Maryland celebration.
After qualifying and posting more than 150 video pitches from Maryland entrepreneurs that were captured during the three-week Pitch Across Maryland tour | celebration, Startup Maryland is proud to announce Vheda Health (Howard County/MCE) and BrinkBit (uBalt, ETC, EAGB, GBC) as Winner and Runner-up, respectively.
back to top 

Montgomery County Executive Ike Leggett is hoping to broaden Montgomery County’s reach in biotech and other high-tech areas as he leads a delegation of two dozen County businessmen and businesswomen to India this month.
Among those joining Leggett are Maryland State Delegate Aruna Miller, Montgomery County Councilmember Nancy Floreen, Montgomery College President Dr. DeRionne P. Pollard, Global LifeSci Development Corporation executive vice president Jonathan Genn, President & CEO of the India-US World Affairs Institute Vinod Jain, and former White House communications director Ann Lewis.
back to top 

If you’re a foreign entrepreneur looking to break into the U.S. market, the State of Maryland wants to help.
On the third floor of a nondescript office building perched on a busy commercial strip in College Park, Maryland, foreign-owned startups can get a boost at the Maryland International Incubator, a first-of-its-kind incubator focused exclusively on foreign companies settling in the United States.
back to top 

A Johns Hopkins astrophysicist will share in a $3 million prize for his discovery that the universe is expanding rapidly, contrary to earlier belief.
Adam Riess, who previously won a Nobel Prize for his work, has been awarded the Breakthrough Prize in Fundamental Physics. Riess shares the award with research partner Brian P. Schmidt of the Australian National University and University of California, Berkeley astrophysicist Saul Perlmutter.
back to top 

Swiss drugmaker Roche said Friday that the Food and Drug Administration approved its drug Avastin as a treatment for ovarian cancer.
Roche said the FDA approved Avastin in combination with chemotherapy as a treatment for recurrent cases of cancer that are resistant to platinum-based chemotherapy.
back to top 

Join us Nov. 20 for the 15th annual Bioscience Day at the University of Maryland, where we will explore “Scientific Advances in Treating Trauma and Disease.”
Registration is free and lunch is provided.
back to top 
 Wednesday, November 19, 2014, 05:00am – 07:00pm Wednesday, November 19, 2014, 05:00am – 07:00pm
Join BioBuzz and our sponsor CRB for another great BioBuzz networking event on November 19th from 5:00 – 7:30 p.m. @ American Tap Room in Rockville, MD. This location is a short walk from the Metro located in the Rockville Town Center.
back to top 
 The MCCC Business Awards Dinner Committee and Chair of the Board Lisa Cines, CPA of Dixon Hughes Goodman LLP invite you to join in the celebration of those who make our economy and community thrive. This annual sold-out event attracts 700+ guests including award winners, sponsors, business leaders, elected and government officials and the media. Join us for a great evening of Meaningful Connections, Commerce and Celebration. The MCCC Business Awards Dinner Committee and Chair of the Board Lisa Cines, CPA of Dixon Hughes Goodman LLP invite you to join in the celebration of those who make our economy and community thrive. This annual sold-out event attracts 700+ guests including award winners, sponsors, business leaders, elected and government officials and the media. Join us for a great evening of Meaningful Connections, Commerce and Celebration.
back to top 

Seniors in the University of Maryland’s Fischell Department of BioEngineering (BioE) design and build devices designed to improve patient outcomes and health care while lowering costs. BioE teams are typically matched with a pair of advisors including a BioE faculty member and a physician from the University of Maryland Medical Center. The teams are assisted by University engineers for fabrication and by business advisors for entrepreneurship. This year, there are 91 students comprising 18 teams of innovators full of fearless ideas.
back to top 

Eighth grader Lily DeBell won the 2014 NFTE National Youth Entrepreneurship Challenge, presented by the MasterCard Center for Inclusive Growth.
DeBell, who is still in middle school, finished ahead of 40 other student entrepreneurs from across the county – almost all of them were either High School or college age students.
back to top 

In a breakthrough in the design of batteries, a research funded by the US Department of Energy has produced a remarkable new prototype battery that just needs 12 minutes to get fully recharged compared to the hours the conventional cells take up to get replenished.
Researchers at the University of Maryland who were involved in this research stated that their new invention can work towards the long sought-for miniaturization of energy storage components. This breakthrough can certainly help towards allowing electric cars to give petrol-powered vehicles a run for their money.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
Notices:
- Findings of Research Misconduct
- Findings of Research Misconduct
- Findings of Research Misconduct
- Notice of Intent to Publish a Funding Opportunity Announcement for Science of Behavior Change: Assay Development and Validation for Self-Regulation Targets (UH2/UH3)
- Notice of Intent to Publish a Funding Opportunity Announcement for Science of Behavior Change: Assay Development and Validation for Stress Reactivity and Stress Resilience Targets (UH2/UH3)
- Notice of Intent to Publish a Funding Opportunity Announcement for Science of Behavior Change: Assay Development and Validation for Interpersonal and Social Processes Targets (UH2/UH3)
- Notice of Intent to Publish a Funding Opportunity Announcement for NIH Science of Behavior Change Resource and Coordinating Center (U01)
Requests for Applications:
- T32 Training Program for Institutions That Promote Diversity (T32)
- (RFA-HL-16-007)
- National Heart, Lung, and Blood Institute
- Application Receipt Date(s): February 18, 2015, September 18, 2015 (resubmissions only), February 18, 2016, September 19, 2016 (resubmissions only); February 18, 2017, September 18, 2017 (resubmissions only) by 5:00 PM local time of applicant organization.
Program Announcements:
- Administrative Supplements for Research on Sex/Gender Differences (Admin Supp)
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
Notices:
- Notice Announcing Frequently Asked Questions (FAQs) for RFA-RM-14-016 “Model Organisms Screening Center for the Undiagnosed Diseases Network (UDN) (U54)
Requests for Applications:
- Stem Cell-Derived Blood Products for Therapeutic Use (R01)
- (RFA-HL-15-022)
- National Heart, Lung, and Blood Institute
- Application Receipt Date(s): February 20, 2015
- Stem Cell-Derived Blood Products for Therapeutic Use: Technology Improvement (R41)
- (RFA-HL-15-029)
- National Heart, Lung, and Blood Institute
- Application Receipt Date(s): February 20, 2015
- Stem Cell-Derived Blood Products for Therapeutic Use: Technology Improvement (R43/R44)
- (RFA-HL-15-030)
- National Heart, Lung, and Blood Institute
- Application Receipt Date(s): February 20, 2015 (Phase I or Fast-Track); February 20, 2016 (Phase I; Phase II; or Fast-Track); February 20, 2017 (Phase II only)
- NHLBI Bench to Bassinet Program Administrative Coordinating Center (U01)
- (RFA-HL-16-004)
- National Heart, Lung, and Blood Institute
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- Application Receipt Date(s): March 17, 2015
- Short-Term Research Education Program to Increase Diversity in Health-Related Research (R25)
- (RFA-HL-16-008)
- National Heart, Lung, and Blood Institute
- Application Receipt Date(s): September 18, 2017
Program Announcements:
- Collaborative Activities to Promote Metabolomics Research (Admin Supp)
- (PA-15-030)
- NIH Roadmap Initiatives
- National Cancer Institute
- Application Receipt/Submission Date(s): Multiple dates, see announcement.
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

The 1776 incubator, which is interested in helping startups break down geographic barriers and collaborating with other incubators and accelerators has announced a partnership with a major physician association — the American College of Cardiology, according to a company statement. The cardiology association will play a role not only in the incubator’s Challenge Cup, but also longer term.
back to top 

The United States has over 5,700 hospitals, and most of them are central to their communities for an obvious reason: They help people get healthier. When I look at these hospitals, I see an untapped resource, a way they could provide greater value to their communities and the country.
Intellectual assets — the ideas and know-how in the heads of clinicians — are vital, intangible resources for most hospitals. They’re equivalent to the research assets at universities. In addition to knowledge and know-how, clinicians working in hospitals are creating ideas for new health care technologies (apps, processes, devices, therapies, drugs) and cost-effective care models, often as part of their response to the value-based care principles of health care reform.
back to top 
 Americans include two health-related issues among the 10 most important problems facing the U.S., according to a recent Gallup survey. Healthcare in general ranked fourth on the list, with Ebola coming in at no. 8. But is Ebola really among the biggest health problems for Americans? Not when we look at the chances of actually being infected. Americans include two health-related issues among the 10 most important problems facing the U.S., according to a recent Gallup survey. Healthcare in general ranked fourth on the list, with Ebola coming in at no. 8. But is Ebola really among the biggest health problems for Americans? Not when we look at the chances of actually being infected.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

November 19
American Tap Room

November 20
Stamp Student Union

November 20
Bethesda North Mariott

December 8
Johns Hopkins Medical Campus

May 7- 8
Bethesda Mariott
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 Thomas Kucharski (IEDC) – Ethan Byler (BHI) – William C. Sproull (IEDC) Thomas Kucharski (IEDC) – Ethan Byler (BHI) – William C. Sproull (IEDC)
BioHealth Innovation, Inc. (BHI) was selected as an International Economic Development Council (IEDC) Excellence in Economic Development Silver Award recipient. BHI is receiving the award in the Public-Private Partnerships category for its work to assemblethe partnership that sponsored DreamIt Health IT Baltimore accelerator program. The award, which recognizes outstanding and innovative development projects that have significantly enhanced the economic revitalization of distressed communities, states, or regions, was formally presented to BHI on Monday, October 20, during the 2014 IEDC Annual Conference in Fort Worth, TX.
“BHI is honored to be acknowledged with this award for our work with DreamIt Health in support of the DreamIt Health Baltimore program in 2014,” said Richard Bendis, BHI President & CEO. “This reinforces the dedication to entrepreneurship and innovation in the biohealth arena that we are constantly striving for in Central Maryland.”
back to top 

Tuesday said its global biologics research and development arm, MedImmune, has agreed to buy privately-held Definiens, which would strengthen MedImmune’s focus on the discovery of novel predictive biomarkers in immuno-oncology.
Definiens is into imaging and data analysis technology, known as Tissue Phenomics, which dramatically improves the identification of biomarkers in tumour tissue.
back to top 

Considering that Emergent BioSolutions (NYSE: EBS ) has regular government contracts and other manufacturing contracts — so regular that it’s willing to give quarterly guidance, which many companies wouldn’t dream of — it was a little surprising to see by how much the biotech beat the guidance it gave three months earlier. Third-quarter revenue came in at $138 million, substantially higher than the guidance of $110 million to $125 million it gave three months ago.
But, as it turns out, this is just an accounting issue. In the third quarter, Emergent BioSolutions set up a collaboration with MorphoSys to develop its prostate cancer drug candidate ES414. Under the terms of the agreement, Emergent BioSolutions got a $20 million upfront payment — the biotech recognized $15.3 million of this in the third quarter. Back out the payment, and Emergent BioSolutions’ revenue falls within its previous guidance, fortunately at the upper-end.
back to top 

Osiris has a history of developing treatments — like Prochymal, the first government-approved stem cell drug — and then selling them to other companies. Its product portfolio is thus diverse, and that is wise when it comes to Grafix, which competes with a number of other advanced wound care products.
back to top 

Ryan Sysko needed someone to push WellDoc’s diabetes management app, BlueStar, into the market. It was time to turn to an outsider to make sure BlueStar successfully sold in Maryland and, eventually, nationwide. The FDA-approved smartphone app helps diabetes patients track blood glucose levels, medications, diet and exercise on a cellphone. It requires a prescription from a doctor.
To take on the task, Sysko, the Baltimore health care technology company’s CEO, called on Kevin P. McRaith. Former vice president of …
back to top 

A year ago Robert Lord and Nick Culbertson were medical students with an idea for a company.
Now their startup Protenus Inc. is doing business with Johns Hopkins Hospital, figuring out how to use $770,000 in seed financing and hiring staff members to get their cyber security system in more hospitals.
back to top 
Cartagenia announced today that it and Qiagen’s bioinformatics business are part of a consortium that has received €1.4 million ($1.7 million) in funding from a European funding initiative to support the development of software tools for personalized genetic analysis of cancer variants.
The Lungcadia program will combine the technologies and expertise of Cartagenia, Qiagen, and the Institute of Pathology at Hannover Medical School and will focus on lung cancer as a disease model, but the partners aim to make the results applicable to other types of cancer. The project has received the funding from the EuroTransBio initiative, which supports biotech collaborations in Europe.
back to top 

The MdBio Foundation, Inc. (MdBio), a nonprofit organization, today announced that it has received a grant of $50,000 from AstraZeneca and its Gaithersburg, Md.-based global biologics research and development arm, MedImmune. The grant will enable the foundation to expand its focus on science, technology, engineering and math (STEM) education and prepare today’s students to become the workforce of tomorrow.
“Our goal is to show students the many possibilities their future can hold with a strong foundation in STEM,” said Brian Gaines, CEO of MdBio. “AstraZeneca’s and MedImmune’s generosity will enable us to expand our programs to ensure that we reach students who can benefit from enhanced educational opportunities. Our state is well-known for its strength in the bioscience market, and we hope to foster the next generation of employees for the companies that call Maryland home.”
back to top 

Dr. William Kirwan, chancellor for the University System of Maryland, announced over the summer that he will be stepping down, but not until his replacement is hired.
He’s lost count of the number of college graduations he’s been a part of since taking the job as Maryland’s education chancellor more than a decade ago.
back to top 

Johns Hopkins University, Biomedical Engineering, CBIDMonday, December 8, 2014 from 3:00 PM to 7:30 PM (EST)Baltimore, MD
Join us December 8th for a review of 16 exciting healthcare design projects at the Johns Hopkins Center for Bioengineering Innovation & Design (CBID). CBID MSE and Undergraduate projects will be presented. This year we’re honored to have as our Keynote Speaker Dr. John Kostuik. Dr. Kostuik is Co-Founder and Chief Medical Officer of K2M Inc, a world leading spine surgery company. The event will also have a Shark Tank competition with a panel of tough judges and real cash prizes. Refreshments will be provided.
back to top 

The University of Maryland earned a gold medal in the International Genetically Engineered Machine competition (iGEM) held in Boston from Oct. 30 to Nov. 3, 2014.
The competition engages student-led teams from universities across the globe to present novel synthetic biology projects that address real-world problems.
back to top 

The National Institute of Standards and Technology and the University of Maryland have collaborated to build a new center that will preform studies on how to store, process and transmit data using quantum architecture.
The Joint Center for Quantum Information and Computer Science is designed to complement basic quantum research being done at an institute run by NIST, UMD and the National Security Agency, NIST said Oct. 31.
back to top 

Chevy Chase-based Wedding Wire, the largest and most trusted online marketplace connecting merchants with engaged couples and party planners, ranked highest of any other Maryland company this year, coming in at number seven with 2013 revenue of nearly $34 million. Also making the Top 10 is Rockville-based CoesterVMS, a leader in the mortgage banking and appraisal industry, coming in at number nine with $14.47 million in revenue in 2013.
back to top 

DAASN is proud to be an Enthusiast sponsor for Tedco’s 4th Annual Entrepreneur Expo and a Major sponser of Startup Maryland.
Startup Maryland Announces Great Eight Finalists and Fan Favorites for 2014 Pitch Across Maryland Tour
Diversity of Industries and Opportunities Defines the Third Annual State-Wide Tour and Celebration of Maryland Innovation and Entrepreneurship.
back to top 

Vheda Health, a digital health company that seeks to help people with chronic conditions, is one of a handful of healthcare startups that have received backing from a commercialization fund from Maryland’s Technology Development Corp. They were part of a list of 15 companies that received $1.5 million. It follows a funding allocation from earlier this year.
The Maryland program allocates funding to companies developing technology products with universities and/or federal labs in the state. To qualify, companies have to be in a collaboration with a federal lab or university, they have to be located in an affiliate incubator company in the state, they have to be involved in one of two Maryland programs — ACTiVATE, an entrepreneurship training program aimed at women, or INNoVATE — or participate in TEDCO’s Rural Business Initiative. The focus is on small businesses so companies must have under 16 employees, or be a university spin-off under five years old or have venture investments under $500,000. More than half of the companies’ employees must work in the state.
back to top 

Business accelerator DreamIt Health Baltimore plans to lay roots in the Inner Harbor.
DreamIt Health Baltimore is negotiating a lease as it prepares to welcome its second class of entrepreneurs in 2015. The accelerator, which is run by Philadelphia-area DreamIt Ventures, operated out of spare office space at a Johns Hopkins building in Fells Point this year. Managing Director Jason Hardebeck hopes to finalize a deal for between 5,000 square feet and 6,000 square feet of space in the coming weeks.
back to top 

Nominations are being accepted for the 2015 FLC Laboratory Director of the Year Awards. Sponsored by the FLC National Advisory Council, this award honors Laboratory Directors who have made maximum contributions to the overall enhancement of technology transfer for economic development. Accomplishments related to the transfer of technology from the federal laboratory to industry—including support of FLC activities, internal accomplishments, industry involvement, and community service—are the primary criteria for the award.
back to top 

As CMS goes, so goes private insurance. That’s perhaps a simplification of how reimbursement rates are developed for healthcare payers, but big payers definitely watch what CMS is doing.
So it’s no small deal that CMS late last week issued new rules that include “significant additional coverage for telemedicine services,” the American Telemedicine Association said in a release.
back to top 

When we think of Google, we think of the company that powers the widely used search engine, and we think of computer programming, engineering, and electrical design. However, recently Google has expanded and moved towards research in medical technology. Just a few months ago, the tech giant partnered with Novartis to license a glucose measuring smart contact lens. The company had also recently bought portions of Calico, an anti-aging research company, and 23andme, a company that provides personal genetic tests. Now, Google aims to develop a wearable diagnostic device to detect cancer and heart attacks through the use of nanoparticles.
back to top 

Let’s face it: pre-Election Night, this year’s race for Maryland governor was pretty underwhelming, the kind where your mother might reveal she’s thinking of voting for Republican businessman Larry Hogan because she remembered his dad to be a nice guy back when he was Prince George’s County executive in the 1970s.
(Hi, Mom! For the record, I don’t know who she ended up voting for.)
back to top 

University of Maryland University College recently swept two divisions of the Maryland Cyber Challenge for the second consecutive year, capturing first place in the College and Professional divisions of the premier Maryland cyber competition held at the annual Cyber Maryland conference in Baltimore.
“This is a tough competition and to beat many great teams just to get to the finals in the college and professional divisions is an amazing accomplishment. We fielded four teams for this competition (two in each division) and all four made it to the final round. That speaks to the quality of our teams, ” said Jeff Tjiputra, chairman of UMUC’s undergraduate cybersecurity program and academic advisor to the cyber competition teams.
back to top 
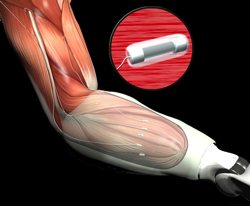
The Pentagon’s technology arm is prepared to invest up to $700,000 in a promising idea in the field of biological sciences and technology. The goal is to turn theoretical concepts into actual products, such as better sensors for prosthetic limbs and techniques to cope with infectious disease outbreaks.
Under a new initiative by the Defense Advanced Research Projects Agency, inventors will be able to send in proposals without having to trudge through miles of red tape as they would have to in traditional government contracts.
Photo Credit: DARPA
back to top 

The National Institutes of Health is challenging science innovators to compete for prizes totaling up to $500,000, by developing new ways to track the health status of a single cell in complex tissue over time. The NIH Follow that Cell Challenge seeks tools that would, for example, monitor a cell in the process of becoming cancerous, detect changes due to a disease-causing virus, or track how a cell responds to treatment.
The challenge aims to generate creative ideas and methods for following and predicting a single cell’s behavior and function over time in a complex multicellular environment – preferably using multiple integrated measures to detect its changing state.
back to top 

Wednesday, November 19, 20146:00 p.m. – 8:00 p.m.
Get out of the office and enjoy yourself! Join Maryland’s business community for an evening of networking and fun at the Tech Council of Maryland’s Fall Cocktail Reception. This reception will be held at the prestigious Congressional Country Club in Bethesda. If you haven’t attended our FEF Fall Reception, come and find out what you’ve been missing.
The reception features an open bar, great food and two hours with no agenda other than networking, seeing old friends and meeting new people. This is the perfect atmosphere for executives and decision makers from the region’s leading companies to come together and share ideas. Everyone is welcome and you don’t need to be a financial executive to attend this event, so register today.
back to top 

The NBIA (National Business Incubation Association) seeks a dynamic new President and Chief Executive Officer. Our ideal candidate will be an exceptional leader with business expertise and a passion for entrepreneurship, as well as the drive, intellect, and professional presence to support and promote business incubation and acceleration.
The incoming President and CEO will report directly to the Board of Directors, and it is critical that this individual be well-versed in the broader national and international entrepreneur support ecosystem (including but not limited to business incubation, acceleration, coworking/emerging startup models, and economic environments – i.e. programs serving rural, urban, and developing economies).
back to top 

iHealth is one of the leading developers of connected health devices, with devices on the market ranging from the BP5 connected blood pressure cuff to the Align wireless glucometer. As we recently highlighted, they have also been at the forefront of integrating the data captured by these devices with the electronic health records used by clinicians.
We had the opportunity to talk with Jim Taschetta, Chief Marketing Officer and Head of Retail Sales at iHealth, about their current work at Duke & Stanford exploring EHR integration, views on integration of Android devices, and more.
back to top 
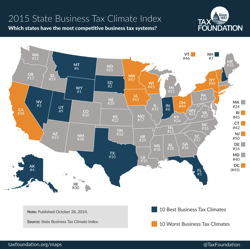
The Tax Foundation’s State Business Tax Climate Index enables business leaders, government policymakers, and taxpayers to gauge how their states’ tax systems compare. While there are many ways to show how much is collected in taxes by state governments, the Index is designed to show how well states structure their tax systems, and provides a road-map to improving these structures.
back to top 

Inspired by a series of new strategic initiatives, BioNJ announced a rebranding today — BioNJ, The Gateway to Health – that reflects an expanded vision and mission directed to fostering a vibrant life sciences ecosystem in New Jersey where science is supported, companies are created, drugs are developed and patients are paramount.
The rebranding is supported by the launch of a revitalized website at www.BioNJ.org that is contemporary in its look and represents the determination of BioNJ to help move the life sciences industry forward.
back to top 

Brain science is taking a hit, according to a recent series of papers published in a special issue of the Cell Press journal Neuron.
“While the disease burden and economic impacts are on the rise, progress in the development of new therapeutics and treatment approaches has appeared to have stalled,” reads an editorial introducing the issue. “Approval for new therapeutics (whether drugs, devices, or other treatment approaches) for nervous system disorders have been declining and most of the treatments we currently have are not disease modifying.”
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

November 12
BWI Airport Marriott

November 19
American Tap Room

November 20
Bethesda North Mariott

May 7- 8
Bethesda Mariott
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Every once and a while you get a reminder that lives are literally at stake in some R&D partnerships. Last Wednesday was one of those days. I was privileged to moderate a panel for the Congressional Technology Transfer Caucus on innovative partnerships fostered by the National Center for Advancing Translational Sciences (NCATS) the newest center/institute at the National Institutes of Health. It was anything but a run of the mill tech transfer session.
We often hear that $2 billion to $5 billion are required to commercialize a new drug, with 14 years or more required for development and a 95% chance of failure. Less well known is that for thousands of serious diseases plaguing humanity only about 500 have FDA approved treatments available. Stark as that seems it’s downright cheery compared to rare or neglected diseases. Of more than 6,500 such ailments only 250 have treatments. While these may be “rare” diseases for many of us, to millions of our friends, families and neighbors each morning brings another day of suffering desperately hoping that someone, somewhere is working on a cure.
back to top 

Paragon Bioservices, Inc. (“Paragon”), a privately held contract research and manufacturing organization whose mission is to accelerate the development and manufacturing of biopharmaceuticals and vaccines, announced today that it has raised $13 Million in a Series A Preferred Stock financing led by NewSpring Capital and Camden Partners.
NewSpring Capital, headquartered in Radnor, PA, invests in dynamic companies, partnering with management teams to accelerate growth and develop their businesses into market leaders. Camden Partners, located in Baltimore, MD, operates private equity funds that provide growth capital to emerging companies in the Technology-Enabled Business Services, Healthcare, and Educational sectors. 1st BridgeHouse Securities and Evergreen Advisors, LLC, Columbia, MD acted as advisors to Paragon Bioservices. All securities transactions were conducted through 1st BridgeHouse Securities, LLC, a member of FINRA and SIPC.
back to top 

New Enterprise Associates is preparing to raise the largest venture capital fund in history, Fortune has learned.
The 37 year-old firm has told investors to expect formal documents by year-end for its fifteenth fund, which is expected to target approximately $2.8 billion. That’s nearly a 10% increase from the $2.6 billion NEA raised for Fund 14 in the summer of 2012, which itself was the industry’s record-holder (just edging out a $2.56 billion fund raised in 2006 by Oak Investment Partners). It also told prospective investors that longtime partner Scott Sandell will be promoted to co-managing partner, alongside Peter Barris.
back to top 

Strand Life Sciences (Strand) representatives will demonstrate new capabilities of the company’s variant interpretation and reporting software, StrandOmics at the Association For Molecular Pathologist (AMP) 2014 Annual Meeting to be held from November 12th to 15th in National Harbor, Maryland. The 20th anniversary meeting’s theme is “Realizing the Dream of Precision Medicine,” with a special address by Dr. Francis Collins, Director of the National Institute of Health. At AMP, Strand will host a workshop on the innovative developments and application of its StrandOmics software, plus new expansion efforts in Personalized Medicine.
back to top 

Baltimore pharmaceutical firm Profectus BioSciences Inc. has received a $9.5 million grant from the Department of Defense for a phase 1 clinical trial of its Ebola vaccine. T
he award is Profectus’ fourth this year — all for work developing and manufacturing Ebola vaccines. The new grant brings the company’s total funding up to at least $49.8 million.
back to top 

The chancellor is stepping down. After 50 years in education, and 12 years as Chancellor of the University System of Maryland, William English “Brit” Kirwan is retiring. He will leave office after his replacement is selected by the Board of Regents.
The search is underway.
During his tenure, Kirwan was hailed for his strategic spending; his “Effectiveness and Efficiency Initiative,” started in 2003-04, has saved the system upwards of $462 million to date. He was also partly responsible for the tuition freezes after the recession. Under him, enrollment in Maryland’s 11 institutions of higher learning increased by 24 percent.
back to top 

When it comes to deadly infections, Ebola is certainly the virus du jour. But in the U.S., the risk of contracting the Ebola virus is minuscule compared to the risk of becoming infected with one of several antibiotic-resistant bacteria, sometimes known as “superbugs.”
back to top 

Novavax Inc.’s singular drug development approach to a possible Ebola vaccine may not only help stop the spread of a future pandemic but also reap major bottom-line rewards.
That’s what CFO Barclay “Buck” Phillips told me this week after the Gaithersburg-based company announced it planned to begin Phase 1 clinical trials by December. Novavax announced this week at the 8th Vaccine and ISV Conference in Philadelphia that it’s the only company targeting the newest strain of the virus which emerged in Guinea this year and has killed thousands in West Africa.
back to top 

For five years, John Eldridge and his team at Profectus Bioscience have developed and tested their Ebola vaccine. First it was on guinea pigs, then monkeys.
At that point, Eldridge realized monkeys weren’t getting sick.
back to top 

Johns Hopkins engineers have invented a lab device to give cancer researchers an unprecedented microscopic look at metastasis, the complex way that tumor cells spread through the body, causing more than 90 percent of cancer-related deaths. By shedding light on precisely how tumor cells travel, the device could uncover new ways to keep cancer in check.
The inventors, from the university’s Whiting School of Engineering and its Institute for NanoBioTechnology, published details and images from their new system recently in the journal Cancer Research. Their article reported on successful tests that captured video of human breast cancer cells as they burrowed through reconstituted body tissue material and made their way into an artificial blood vessel.
back to top 

Montgomery County has chosen two partners to begin planning a “kitchen incubator” to provide business training and commercial kitchen space to budding local culinary entrepreneurs.
The county is partnering with Union Kitchen, which runs a for-profit commercial kitchen space in the District, and Streetsense, the Bethesda-based design and development firm, to plan how the incubator will be set up.
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
- Notice to Extend PAR-12-033 “Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Etiology, Diagnosis, Pathophysiology, and Treatment (R21)” by an Additional Funding Cycle
(NOT-OD-15-007) Office of Research on Women’s Health
- Notice of Clarification of 4-Year Limit of Postdoctoral Research Eligibility for K99 Applicants for PA-14-042 NIH Pathway to Independence Award (Parent K99/R00)
(NOT-OD-15-013) Office of the Director, NIH
- Notice of Revised NIH Definition of Clinical Trial
(NOT-OD-15-015) National Institutes of Health
Requests for Applications (RFAs):
- Pediatric HIV/AIDS Cohort Study (PHACS) Coordinating Center (CC) (U01)
(RFA-HD-15-027)
Eunice Kennedy Shriver National Institute of Child Health and Human Development
Application Receipt Date(s): January 02, 2015
- Pediatric HIV/AIDS Cohort Study (PHACS) Data and Operations Center (DOC) (U01)
(RFA-HD-15-029)
Eunice Kennedy Shriver National Institute of Child Health and Human Development
National Institute on Alcohol Abuse and Alcoholism
National Institute of Allergy and Infectious Diseases
National Institute on Drug Abuse
National Institute on Deafness and Other Communication Disorders
National Institute of Dental and Craniofacial Research
National Institute of Mental Health
National Institute of Neurological Disorders and Stroke
Office of AIDS Research
Application Receipt Date(s): January 02, 2015
- NIH Director’s Early Independence Awards (DP5)
(RFA-RM-14-004)
NIH Roadmap Initiatives
National Institute of Dental and Craniofacial Research
Application Receipt Date(s): January 30, 2015
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Startup Maryland (www.startupmd.org) today announced the Great Eight (8) Finalists and three (3) Fan Favorites from the 2014 Pitch Across Maryland celebration.
After assembling more than 150 video pitches from Maryland entrepreneurs that were captured during the three-week Pitch Across Maryland tour | celebration, Startup Maryland is proud to announce the eight finalists as well as the three startups with the most view-votes. Companies from both categories are listed below (with tour stop) and link to their pitch video:
back to top 

Inspiring the scientists of the future is something that MedImmune, the global biologics research and development arm of AstraZeneca, takes very seriously. Therefore the Society of Biology’s ‘Big Biology Day’ – a one day, free-to-attend event aimed at engaging the public in scientific issues and research activities – has become a permanent fixture in MedImmune’s calendar. This year’s event, which took place on Saturday 18th October at Hills Road Sixth Form College, was attended by over 1,000 people of all ages and was the third Big Biology Day to be held in Cambridge. As usual, it formed part of National Biology Week, the Society of Biology’s annual celebration of the biosciences and was held in collaboration with the University of Cambridge Science Festival.
back to top 

Johns Hopkins University is the 11th best university in the world, according to the first ever Best Global University rankings published by U.S. News & World Report on Tuesday. The rankings use different criteria than those used for the annual “best colleges” list, on which Johns Hopkins ranked 12th last month.
The new rankings include 500 universities in 49 countries. Sixteen of the top 20 universities are in the U.S., including Harvard, which tops the rankings.
back to top 

Tuesday, November 18, 2014 | 6:00-8:30 p.m.
Silver Spring Civic Center-One Veterans Plaza
GPS Address: 8525 Fenton Street, Silver Spring
Meet Israeli entrepreneurs working in Maryland and Maryland companies trading with Israel at the MIDCs fourth annual Showcase of Maryland/Israel Business.
Featuring keynote speaker, Jeremy Bash,Founder and Managing Director, Beacon Global Strategies and former Chief of Staff, Department of Defense and CIA
back to top 

American Tap Room – 36 Maryland Ave Rockville, MD 20850
Join BioBuzz and our sponsor CRB for another great BioBuzz networking event on November 19th from 5:00 – 7:30 p.m. @ American Tap Room in Rockville, MD. This location is a short walk from the Metro located in the Rockville Town Center.
CRB is a global consulting, design and construction services firm that relentlessly pursues and delivers success for their clients in advanced science and technology industries. 2014 marks CRB’s 30th year in which they have grown from a single three-person office, to a team of 600+ in 12 offices serving clients throughout the world with Rockville, MD being their latest expansion.
back to top 

Maryland Department of Health and Mental Hygiene officials announced early Tuesday that the patient tested negative for Ebola. The department said that person will continue to receive the appropriate treatment.
A patient who was being isolated at the University of Maryland Medical Center in Baltimore for Ebola testing Monday evening does not have the deadly virus, officials announced Tuesday.
back to top 

Advent Life Sciences today announced the closing of Advent Life Sciences Fund II (ALSF II), a £145.5M (USD 235M) venture capital fund raised to seed and build life sciences companies in the UK, Europe and the US. The Fund will back entrepreneurs and early-stage and mid-stage companies with the potential to deliver first- or best-in-class breakthrough products for unmet medical needs. The Fund, which quickly exceeded its target, was raised entirely from independent financial investors including funds-of-funds, pension funds, and family offices.
“We thank our returning and new LPs for the strength of their support, which allowed us to raise the commitments for this Fund in a matter of weeks” said Raj Parekh, General Partner. He added “The interest from LPs and demand for the Fund, particularly in current markets, is in large part a recognition of the scientific and medical entrepreneurs, CEOs and Management teams whose commitment, vision and energy is enabling our portfolio companies to bring important medical innovations to patients. It is a privilege to work with them.”
back to top 

Two new studies in the New England Journal of Medicine rocked the world of celiac research, both proving that scientists have a ways to go in their understanding of celiac disease, which affects about 1% of the population, whether they know it or not.
One Italian study wondered if the age at which gluten is introduced into the diet could affect a person’s likelihood of developing the autoimmune disease—so they kept gluten away from newborns for a year. To the shock of the researchers, delaying exposure to gluten didn’t make a difference in the long run. In some cases it delayed the onset of the disease, but it didn’t stop people from developing the disease, for which there is no cure.
back to top 

Cancer’s become a core area of venture capital interest, particularly given the rise of personalized medicine. But in a cluttered marketplace it’s tough to differentiate the worthy from the chaff. Here are some observations from a panel of investors said at the Cleveland Clinic Medical Innovation Summit Monday:
back to top 

The San Francisco-based digital health accelerator Rock Health has raised a large third round of funding and says it will boost its investments in new portfolio companies to (up to) $250,000 each.
The new funding round was led by Bessemer Venture Partners and Kaiser Permanente Ventures, with participation from KPCB, Mayo Clinic, Montreux Equity Partners, and Great Oaks Ventures.
back to top 

I’m so used to hearing bullish projections on digital health, it’s refreshing when someone contradicts that assessment. Maybe contradict is the wrong word. But Thomas Rodgers, who joined McKesson Ventures last month after a couple of years with Cambia Health Solutions, thinks it will take a lot longer for the technology to enjoy mainstream adoption.
“I think it will be 15-20 years until it is intertwined with medical care. It will take a shift away from fee-for-service and it will also take generational change. Millennials who grew up with technology will need to start getting sick.”
back to top 

In the decade after the founding of the BioCrossroads initiative, money spent on life sciences research and companies more than doubled, to more than $25 billion, according to a new report released Thursday by the Indianapolis-based life sciences business development group.
That infusion of money—much of which came from out of state—has helped Indiana companies and universities increase the number of life sciences patents, technology licenses, startups and venture capital deals faster than the rest of the nation, according to the report.
back to top 

The Hong Kong scientist who invented a simple blood test to show pregnant women if their babies have Down syndrome is now testing a similar technology for cancer.
Yuk Ming “Dennis” Lo says screening for signs of cancer from a simple blood draw could cost as little as $1,000. The test works by studying DNA released into a person’s bloodstream by dying tumor cells.
back to top 

New York Medical College will inaugurate its biotechnology incubator Wednesday.
The multimillion-dollar BioInc@NYMC at 7 Dana Drive in Valhalla will offer space to scientists, entrepreneurs and biotech firms.
back to top 

As Penn prepares for the ceremonial groundbreaking of the Pennovation Center on Friday, Drexel University’s Innovation Neighborhood is still searching for a master developer.
Both universities are pushing to become incubators of commercial enterprise and educational advancement. Since purchasing a 23-acre site on Grays Ferry Avenue in 2010, Penn has been working to develop the Pennovation Center — a three-story, 52,000-square-foot complex that will serve as a hub for research and business ventures. In addition to the ceremonial groundbreaking on Friday, Penn President Amy Gutmann will host a series of “Pennovation Talks” at the South Bank campus.
back to top 

International VC firm SOSVentures is capitalizing on the now buzz worthy biotech investment trend with the creation of IndieBio, the first accelerator to focus on just life sciences.
Y Combinator raised a few eyebrows when it accepted five biotech companies out of the 80 startups in its program this last summer. That was a first for the Silicon Valley accelerator. But IndieBio partners tell us they were already thinking along those lines when Y Combinator started making in-roads with those life sciences startups.
back to top 

The average board of directors in the biotech world is roughly 90% male, according to a new analysis, and more than half of all industry boardrooms host no women whatsoever, striking numbers that illustrate a sector that struggles with diversity.
Liftstream, a recruitment services outfit, analyzed nearly 1,150 life sciences companies in the U.S. and EU, finding that biotech’s boardrooms tend toward Y chromosomes. Among drug developers with fewer than 1,000 workers, women held just 10% of available board seats, and fewer than 4% had female chairs. C-suites didn’t fare much better, as women accounted for fewer than 25% of leadership teams across the industry.
back to top 

UBI Index recognizes top performing business incubators from all over the world. This time we take a deeper look in 5 regional areas starting with Europe and then moving on to North America, South America, Asia+Oceania and ending with Africa. It is our pleasure to present to you:
The Regional Top Performing University Business Incubators of 2014
The rankings of each region will be announced every Tuesday at http://ubiindex.com/rankings with start on November 4th and four weeks forward. Stay tuned!
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

November 5
Johns Hopkins University Montgomery County Campus

November 12
BWI Airport Marriott

November 19
American Tap Room
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

Sucampo Pharmaceuticals Inc. on Thursday named Peter Kiener — whose past roles includes CEO of Zyngenia and head of biologics R&D for MedImmune — as its chief scientific officer.
Kiener, a heavyweight research hire, worked alongside Sucampo CEO Peter Greenleaf at MedImmune, where Greenleaf served as president until early 2013. Kiener then departed to launch Zyngenia Inc., a New Enterprise Associates-funded biotech focusing on antibody-based therapeutics, which he departed last year.
back to top 

Qiagen has announced the introduction of expanded functionality for its range of bioinformatics workflow solutions.
New capabilities for the Ingenuity Variant Analysis and CLC Cancer Research Workbench solutions were unveiled at the American Society of Human Genetics annual meeting, which recently concluded in San Diego.
back to top 

Tuberculosis is both tough to treat and medically cumbersome to manage. Under directly observed therapy, a medical professional has to watch a patient take his or her medication for at least six months. A Baltimore health IT startup may help lighten that load.
The Baltimore City Health Department is launching a pilot with emocha Mobile Health’s app miDOT, according to a release from the Highlandtown firm.
back to top 

The U.S. government invested $440 million in three vaccine plants in the U.S. in 2012 with the proviso that if something like a pandemic occurred, it could call on them to produce drugs that it required. With Ebola spreading, those calls have now been made.
The Ebola crisis has prompted the Biomedical Advanced Research and Development Authority (BARDA) to ask the three plant owners–Novartis ($NVS), GlaxoSmithKline ($GSK) and Emergent Biosolutions ($EBS) and their partners–to tell it what it would take for them to produce ZMapp, an experimental drug currently being produced through a novel approach using tobacco plants. BARDA wants detailed timetables and budgets for making ZMapp, Reuters reports. They are supposed to respond by Nov. 10.
back to top 

Strategic Adviser for BioHealth Innovation Inc.
Tania Fernandez came to the biotech initiative through the venture capital world, where she worked in Silicon Valley and spent 16 years managing investments in companies.
What is the biggest way your day will change in your new role? My day won’t really change. I am a classified workaholic. I will continue to do what I have always loved doing: working with entrepreneurs, building companies and looking for good investment opportunities in the biotech/life sciences/health care sectors. BioHealth Innovation has built a very commendable ecosystem for startups, and I look forward to the opportunity to build out that ecosystem with them as we get ready to fund raise for the BioHealth Gap Fund.
One thing you wish everyone knew about your job? I founded DreamCatcher Ventures after having spent more than 15 years in hard-core academics and a decade in venture capital financing. I have watched people get burnt either because they were not grounded enough (which translates into operational weakness) or had their head way above the clouds (which translated to being unrealistic about long-term success and sustainability of business models). I created DreamCatcher Ventures with a simple goal to “Build the foundations for dreams.” That’s what I want people to know about my job. This quote by Henry David Thoreau, which is the tagline for DreamCatcher Ventures, says it all: “If you have built castles in the air, your work need not be lost; that is where they should be. Now put the foundations under them.” For entrepreneurs there is a fine line between being stubborn and being persistent, an even finer liner between being a visionary and having unrealistic expectations. In my day-to-day job, I work with clients to bridge that. It is great to dream but you need to have your feet planted on the ground. I believe in the power of dreams combined with the excellence of execution.
back to top 

Colleen Worthington has been working at this university for more than 24 years. It wasn’t until this year, however, that she received her first promotion.
“No matter how much better I got or more I contributed, it was the same title from 1990 until last [semester],” Worthington said.
back to top 

Italian drugmaker Sigma-Tau is in advanced talks to sell part of its Italian operations to domestic peer Alfa Wassermann that would create an over-the-counter (OTC) powerhouse, several sources familiar with the situation said.
Sigma-Tau is working with Milan-based Four Partners, an advisory firm led by Sigma board member Guido Tugnoli, said the sources, who declined to be identified because the matter is private.
back to top 

NewLink Genetics Corp (NLNK.O) said it entered into an agreement with Roche Holding AG (ROG.VX) to develop NewLink’s cancer immunotherapy, making the Ebola vaccine developer eligible to receive over $1 billion in milestone payments.
NewLink’s shares jumped nearly 30 percent before the bell on Monday.
back to top 

Swiss pharmaceutical group Roche hopes to obtain fast-track US approval for a rapid diagnostic test for Ebola, its director general said in an interview published Sunday, amid the worst-ever outbreak of the killer disease.
The diagnostic test is ready for use in scientific research but the company now wants to register it for clinical use.
back to top 

Pharma giant GlaxoSmithKline (GSK) might plan an initial public offering (IPO) of a minority stake in ViiV Healthcare, a global business focused on developing treatments for HIV, reports FierceBiotech.
Created five years ago, ViiV is GSK’s majority-owned joint venture with Pfizer and Shionogi. In the past 18 months, the Food and Drug Administration (FDA) approved two new HIV treatments from ViiV: Tivicay (dolutegravir) and the combo-tablet Triumeq, which includes Tivicay. These successes come at a time when GSK is planning to slash nearly $1.6 billion from its annual budget.
back to top 

It’s no secret that Washington has a funding problem, with lawmakers and federal departments currently operating under unprecedented fiscal constraints.
It’s a very different story for the city’s entrepreneurs.
back to top 

Sucampo Pharmaceuticals, Inc. (Sucampo) (Nasdaq:SCMP), a global biopharmaceutical company, and Takeda Pharmaceutical Company Limited (Takeda) today announced that on October 17, 2014, they entered into a global license, development, commercialization and supply agreement for AMITIZA® (lubiprostone). Through this agreement, Takeda expanded its exclusive rights beyond the United States (U.S.) and Canada to further develop and commercialize AMITIZA in all global markets, except Japan and the People’s Republic of China.
“Takeda is committed to being a patient and customer-centric organization, making quality health products available to the patients who need them. Through this agreement, AMITIZA can now be made available to patients worldwide,” said Shinji Honda, Senior Managing Director and Corporate Strategy Officer. “Takeda forms partnerships to advance science and to provide innovative treatment options for patients, and this global agreement is an excellent example. This global collaboration leverages the expertise we have established through our gastroenterology portfolio of products.”
back to top 

Baltimore biopharmaceutical company Profectus BioSciences Inc. has received a three-year, $8.5 million grant from the U.S. Army for work on an Ebola vaccine.
Profectus will share the grant with the Galveston National Laboratory at the University of Texas Medical Branch at Galveston. The grant is Profectus’ second in recent days. The company also announced a $5.8 million grant from the Biomedical Advanced Research and Development Authority (BARDA) to conduct safety studies of the company’s VesiculoVax, a potential Ebola vaccine.
back to top 

The University of California recently announced its entry into the venture capital arena with a $250 million commitment to spinning promising technologies out of its top-notch, 10-campus system. Perhaps every major research university in the US — collectively, recipients of over $40 billion in federal research funding yearly, not to mention the inflows of corporate research funds — wishes to emulate Stanford’s success in capitalizing on the market successes of university-developed technology (think Google).
back to top 

As a partner at Norwest Venture Partners (NVP), Casper de Clercq has seen countless digital health startups try to get solutions off the ground. He’s also seen many fail.
According to de Clercq, 60 to 70 percent of digital health startups are likely to fail because they are unclear about their go-to market strategy, and don’t have a good understanding of who’s ultimately going to pay for their product.
back to top 

New initiative will support networks that help doctors access information and improve health outcomes
Health and Human Services Secretary Sylvia M. Burwell today announced an initiative that will fund successful applicants who work directly with medical providers to rethink and redesign their practices, moving from systems driven by quantity of care to ones focused on patients’ health outcomes, and coordinated health care systems. These applicants could include group practices, health care systems, medical provider associations and others. This effort will help clinicians develop strategies to share, adapt and further improve the quality of care they provide, while holding down costs. Strategies could include:
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Notice of Special Accommodations for Submission and Reporting Requirements for Program Directors/Principal Investigators Responding to the West Africa Ebola Outbreak
(NOT-OD-15-010) Office of the Director, NIH
Notice to Extend the Response Date for NOT-OD-14-128 “Request for Information (RFI): Consideration of Sex As a Biological Variable in Biomedical Research”
(NOT-OD-15-012) Office of the Director, NIH
Reminder: NIH Requires the Research Performance Progress Report (RPPR) for All Type 5 Progress Reports
(NOT-OD-15-014) Office of the Director, NIH
Notice of Participation of the National Institute on Drug Abuse in PA-14-334 “Advancing Interventions to Improve Medication Adherence (R01)”
(NOT-DA-14-052)
National Institute on Drug Abuse
NHLBI Announces Frequently Asked Questions (FAQs) for RFA-HL-15-015 “Multi-Site Clinical Trials for the Pulmonary Trials Cooperative (U01)” and RFA-HL-15-016 “Network Management Core (NEMO) for the Pulmonary Trials Cooperative (U01)”
(NOT-HL-14-240)
National Heart, Lung, and Blood Institute
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Earlier this year, the Secretary of the Department of Health and Human Services (HHS) launched the HHS IDEA Lab. With it, we unveiled a consolidated structure for the innovation activities at the Department of Health and Human Services, flashy new branding and a website. But when we launched, we weren’t totally clear on what the main message for the HHS IDEA Lab was, and over the past 6 months we heard the question – what is the HHS IDEA Lab all about? So we have looked at ourselves, focused on what your needs are to solve problems, become an entrepreneur, or just learn new skills, and have clearly defined what the HHS IDEA Lab is.
back to top 

In early drug discovery, you need a starting point, says Northeastern University associate professor of chemistry and chemical biology Michael Pollastri.
In a new research paper published Thursday in the journal PLOS-Neglected Tropical Diseases, Pollastri and his colleagues present hundreds of such starting points for potentially treating Human African trypanosomiasis, or sleeping sickness, a deadly disease that affects thousands of people annually.
back to top 

The technology for creating new tissues from stem cells has taken a giant leap forward. Two tablespoons of blood are all that is needed to grow a brand new blood vessel in just seven days. This is shown in a new study from Sahlgrenska Acadedmy and Sahlgrenska University Hospital published in EBioMedicine. Just three years ago, a patient at Sahlgrenska University Hospital received a blood vessel transplant grown from her own stem cells.
Suchitra Sumitran-Holgersson, Professor of Transplantation Biology at Sahlgrenska Academy, and Michael Olausson, Surgeon/Medical Director of the Transplant Center and Professor at Sahlgrenska Academy, came up with the idea, planned and carried out the procedure.
back to top 
Regression models, Monte Carlo simulations, and other methods for predicting what’s around the corner have been in use for decades. It’s only recently, though, that advances in information technology have made it possible for predictive tools to access and manipulate big data, and to do so continuously — accelerating the generation of insights, and opening up opportunities to anticipate issues with unprecedented precision. Think of the colleges that are increasingly able to identify students at risk of dropping out and intervene before they do. Or lenders’ enhanced abilities to gauge credit risk. Energy, agriculture, insurance, retail, human resources — no industry is unaffected. But nowhere is the potential of this new era of opportunity more apparent and exciting than it is in health care.
back to top 
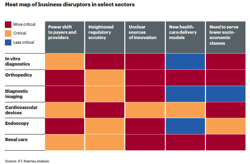
It’s not just the FDA that is making life difficult for medical device companies. Executives are having to follow sales opportunities as medical care shifts out of hospitals into homes and physician offices. They are having to revamp their entire business model to survive in the new world of the ACA.
A.T. Kearney has identified the five forces that are forcing the device industry to evolve in this new report: Medical Devices: Equipped for the Future? In addition to spelling out the threats, the analysts have a guide for how to start building a new business model.
back to top 

Stapling up skin post-surgery is pretty much the norm to quickly seal up wounds, but it runs a risk of infection and injury from the extra damage to already sensitive skin.
Bay Area startup ZipLine Medical has developed a non-invasive but suture-like alternative that it’s positioning as a quicker, simpler and more desirable way to close small surgical wounds. To boot, clinical trials have shown the method decreases both infection likelihood as well as scarring. The company just closed a $5.7 million extension to its Series C financing round, led by a new venture firm in Shanghai called China Materialia that wants to expand the technology there.
back to top 

Massachusetts General Hospital and MIT have formed a $3 million strategic alliance in an attempt to address three “major challenges” that persist in healthcare: improving diagnoses, developing new approaches to prevent and treat infectious diseases and developing more accurate methods of diagnosing and treating neurodegenerative and psychiatric diseases.
The alliance, officials said, will add further heft to already existing efforts between individual collaborations between the two institutions, particularly as they relate to development of diagnostic tools and therapies.
back to top 

Roche plans to spend around $3bn updating and expanding its Basal site, home to the Swiss company’s headquarters, over the next 10 years.
The company is set to build a new R&D centre for 1,900 employees and an office building for 1,700 employees, and will also upgrade its existing infrastructure.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 28
British Embassy

November 5- 6
FLCVirtual

November 6
IBBR

November 8
University of Maryland, College Park

November 20
Bethesda North Mariott
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation clients Alex Lai (Speed BioSystems), Matthew Mulvey (Benevir), David Cetlin (MockV Solutions), and BHI Entrepreneur-in-Resident Ram Aiyar meet with Bahija Jallal.
Dr. Bahija Jallal is Executive Vice President, MedImmune, responsible for biologics research, development and clinical activities. As part of AstraZeneca’s Senior Executive Team, Bahija is tasked with advancing the biologic organisation’s pipeline of drugs targeting cancer, infections, respiratory and inflammatory diseases, cardiovascular and gastrointestinal disorders and pain. (Astrazenica Bio)
back to top 

Biopharmaceutical company Emergent BioSolutions could begin manufacturing an experimental Ebola drug at its Baltimore facility.
Rockville-based Emergent is one of three advanced laboratories asked by the Biomedical Advanced Research and Development Authority (BARDA) to submit a plan for manufacturing ZMapp. The drug has been used among infected health workers in Africa but supplies have run out. BARDA will select one or more of the labs to make more of the drug.
back to top 
 Summary of Position: Summary of Position:
The Director, Regulatory Affairs is the regulatory representative on the Product Development Teams providing regulatory support and advice. The Director will facilitate regulatory strategy development and implementation, take the lead on regulatory submissions, and author, review, and coordinate quality submissions to regulatory agencies. The Director will prepare for meetings, teleconferences, and other communications with FDA, educate teams regarding regulatory risks and implications for strategy and product development activities, and utilize technical knowledge and effectively apply regulations and guidelines to the product development process.
back to top 
Join us in welcoming two new interns to our team!
|
Vanessa Sorto
Accounting and Human Resources Intern
Vanessa Sorto joined BHI on September 2014 as an accounting and human resources intern. She is responsible for supporting the director of finance and human resources in assembling monthly expense reports for BHI senior staff, reconciling bank accounts, preparing monthly financial reports, assisting in audit preparation, and maintaining payroll. She is currently pursuing a bachelor’s degree at The University of Maryland, College Park in Accounting and Information Systems. She is a Hillman Entrepreneur scholarship recipient.
|
 |
|
Kurt Herzog
Business & Research Analyst
Kurt Herzog joined BHI in 2014 as a Business & Research Analyst. He assists BHI’s client companies with market entry strategies for their products by engaging with clients to understand current products, researching existing and potential markets, and analyzing business strategy. His work includes developing executive summaries, investor slide decks, tailored presentation materials, and creating financial models for presentation to investors.
Kurt also works with Tania Fernandez on the BioHealth Gap Fund, a $50 million fund announced October 2014 in partnership with the EAGB to support early-stage therapeutics, medical device, diagnostics, and health IT companies in central Maryland. Kurt has experience in investment banking, international micro-lending, renewable energy project finance and project development, forest carbon sequestration monitoring, institutional investing in hedge funds, and working for a non-profit startup accelerator. Kurt holds a BA in Economics and Environmental Studies with a Natural Science concentration from Bowdoin College. He also completed substantial coursework in chemistry.
|
 |
back to top 

Don’t miss the third annual full-day Innovation 2 Commercialization (I2C) conference featuring three informative plenary sessions to help launch, commercialize and fund YOUR technology to make your business venture profitable!
Hear panels on innovation, commercialization and financing featuring speakers from MedImmune, Brain Sentry, Naval Research Lab and more. Plus, learn about the “Amplimmune Story” and enjoy lunch as you discuss issues affecting your innovation or small business with a subject matter expert from a federal lab, university tech transfer office, venture capital firm, business service organization and others who serve as your table host.
back to top 

Join the BioBuzz networking group at our next free event. Along with 100’s of the region’s bioscience workers, you too could be making new connections, getting jobs and helping to build a stronger, more connected industry through the BioBuzz community!
The event is always free and we offer free drinks to the first 50 to 100 who arrive depending on how much we’ve been sponsored on a given month. People from all around the the region are coming out each month for this unique and welcoming monthly happy hour. It’s a great place to meet up with coworkers past and present, make new connections or just catch up on the latest industry gossip If you’re new and haven’t yet made it out to an event, then we hope that you’ll join us this month and see what all the Buzz is all about.
back to top 

One of the two Ebola-infected Dallas nurses was admitted to a National Institutes of Health biocontainment unit in Bethesda on Thursday, just as researchers at the University of Maryland School of Medicine in Baltimore are beginning the first human trials of an Ebola vaccine in Africa.
Dr. Myron Levine, a researcher at the medical school, is a member of the international medical group leading the efforts to end the outbreak. Ebola has infected more than 8,000 people since the first reported case in March, according to the Centers for Disease Control and Prevention.
back to top 

Applications are now open for DreamIt Health’s second Baltimore cohort.
DreamIt Ventures will accept applications through Dec. 5, according to a DreamIt blog post. The first cohort last year included startups like Protenus, Aegle and Quantified Care.
back to top 

ASTRAZENECA has announced a four-way collaboration which will strengthen the pharmaceutical giant’s link with Cambridge University.
The firm, together with its biologics research and development arm MedImmune, has agreed the new collaboration as it prepares to move its global headquarters to the city. It builds on an existing strategic partnership between the organisations.
back to top 

The Tech Council of Maryland (TCM), Maryland’s largest technology trade association for life science and technology, has been awarded a three-year, $225,000 federal grant designed to help job seekers gain skills in the growing fields of cyber technology and cyber security.
The grant is part of a $15 million federal investment in the Cyber-Technology Pathways Across Maryland (C-PAM) Consortium, spearheaded by Montgomery College. The consortium, which is comprised of 14 community colleges and trade associations, aims to prepare women and other underrepresented populations for jobs in the rapidly growing cyber industry.
back to top 

Sucampo Pharmaceuticals has announced that it signed an amendment to the existing collaboration and license agreement (Collaboration Agreement) covering the United States and Canada for AMITIZA(R) (lubiprostone) with Takeda Pharmaceutical Company Ltd. The amendment includes various modifications to the Collaboration Agreement including the extension of the current term, minimum commercial investment during the current term and various governance changes allowing Takeda additional flexibility in commercializing AMITIZA.
During the extended term, which will begin on January 1, 2021, Takeda will split with Sucampo the gross profits of branded AMITIZA for any dosage strength and form for the existing indications in the U.S. and Canada. In addition, on April 1, 2015 Takeda will no longer reimburse Sucampo for the product details made by Sucampo sales representatives to healthcare professionals as well as other ancillary costs of the sales force.
back to top 

As the tech industry falls back in love with urban America, Joel Marcus, landlord to the booming biotechnology business, is perfectly positioned to strike it rich.
In Manhattan, overlooking the East River and sandwiched between New York University Langone Medical Center and Bellevue Hospital, two gleaming 16-story towers act as beacons to the booming biotech sector.
Completed in 2010, the 310,000-square-foot Alexandria Center for Life Science is already 100% occupied. It contains a hub for Eli Lilly’s cancer business and a Pfizer PFE -1.69% lab dedicated to exploring leads generated by academic researchers. The second building, some 410,000 square feet of labs and office space, was finished in January and is already 60% full. Roche, the anchor tenant, says moving 250 clinical trial specialists there from Nutley, N.J. has resulted in 25 new collaborations with charities, biotechs and New York hospitals. There’s also an accelerator for startups, an enviro-friendly garden and fancy restaurants to feed all those scientists.
back to top 

Becton Dickinson (NYSE: BDX) has acquired GenCell Biosystems, a privately-held Irish biotech company that has developed proprietary technologies that address key biological analysis protocols – from library preparation of Next Generation Sequencing (NGS) to genotyping for agricultural applications.
“We are excited with the GenCell Biosystems acquisition as it provides BD entry into the Next Generation Sequencing market, a fast-growing segment with the potential to have a significant impact on healthcare,” said Linda Tharby, Group President, BD. “The acquisition gives BD access to the NGS market with a differentiated platform that will provide a base to further grow our genomics offerings.”
back to top 

QIAGEN N.V. (NASDAQ: QGEN)(Frankfurt Prime Standard: QIA) announced today that its business area, QIAGEN Bioinformatics, has been named by Genomics England (GeL) an assessment winner for the UK100K project, along with other life science technology providers. The company will be providing project researchers access to Ingenuity® Variant Analysis™, a powerful web-application that allows users to rapidly identify and prioritize disease causing genetic variants using advanced analytics powered by published biological evidence and large numbers of sequenced genomes.
back to top 

Bicycle’s founding venture capitalist (VC), Atlas Ventures joined forces this month with SV Life Sciences and three corporate VCs – Novartis (NVS) Venture Fund, GlaxoSmithKline’s (GSK) SR One and Astellas Venture Management to invest $32m (€25m) in the Cambridge-based biotech company.
Bicycle Therapeutics was established in 2009 as a spin-off of the Medical Research Council Laboratory of Molecular Biology (Cambridge), based on the work of the scientific founders Sir Gregory Winter and Professor Christian Heinis from Trinity College, Cambridge. Winter, a renowned scientist in the biotech industry, also founded Domantis and Cambridge Antibody Technology. Founded in the 80s, Cambridge Antibody Technology was a pioneer in British biotechnology, and was listed on the UK stock exchange in 1997, raising £43m. In 2006, AstraZeneca bought the company.
back to top 

A $5 million Innovation Challenge Fund (ICF), to encourage and support academic groups and small companies in collaborative efforts in the development of bioelectronic medicines, has been announced by GSK. This funding programme is in addition to the company’s prior commitment of a $1 million award (December 2013), for the team that first solves the GSK Bioelectronics Challenge.
Bioelectronic medicine is focused on producing miniaturised, implantable devices that could be programmed to read and correct electrical signals passing along the nerves of the body, to treat disorders as diverse as inflammatory bowel disease, arthritis, asthma, hypertension and diabetes. The ICF funded work and the Innovation Challenge’s winning entry will be made freely available to the global research community.
back to top 

Venture capital investments in Maryland are still working their way back up, according to a report released this week by PricewaterhouseCoopers and the National Venture Capital Association.
In the third quarter of 2014, venture capitalists spent $89 million, up 34 percent from the second quarter’s $66.5 million, but far off the third quarter number from last year, which hit $142 million, according to the report.
back to top 
In the Boston-versus-New York rivalry, the Red Sox and the Yankees were also-rans this baseball season. Now, Massachusetts and New York are in another battle for the No. 2 spot: U.S. venture capital investment.
New York is leading Massachusetts in total venture capital invested so far this year, which if it held up would be the first time the Empire State edged out its East Coast rival and took second place behind industry leader California since at least 1992.
back to top 

A BIOTECHNOLOGY company founded by Limerick man Kieran Curran has been snapped up for $150m by a US firm.
GenCell Biosystems, a company based in Raheen with offices in the US, was acquired late last week by Becton Dickinson, a company listed on the New York Stock Exchange.
back to top 
ATCC, the premier global biological materials resource and standards organization, announces the release of ATCC® Minis to support quality control (QC) testing in pharmaceutical and industrial labs, during the PDA 9th Annual Global Conference on Pharmaceutical Microbiology in Bethesda, MD, Booth # 304.
Healthcare, personal care product, and cosmetic manufacturers are required to test the bio-burden and sterility of their products and production environments to ensure consumer safety. Global alignment and harmonization of microbial testing requirements among the United States Pharmacopeia (USP), Japanese Pharmacopeia (JP), and European Pharmacopeia (EP), have resulted in the need for consistent and reliable control organisms at less than five passages from the ATCC reference stock for reproducible results.
back to top 

A jaw-dropping report released by the World Health Organization on October 14, 2014 reveals that 1 in 20 Ebola infections has an incubation period longer than the 21 days which has been repeatedly claimed by the U.S. Centers for Disease Control.
This may be the single most important — and blatantly honest — research report released by any official body since the beginning of the Ebola outbreak. The WHO’s “Ebola situation assessment” report, found here, explains that only 95% of Ebola infections experience incubation within the widely-reported 21-day period.
back to top 

I joined J&J Consumer Companies about four years ago to start its Digital Center of Excellence. Our role initially was to build capabilities and develop strategy that served multiple brands in multiple regions, so I did a landscape overview to help develop the approach. What I saw was that we had hundreds of different websites and digital platforms that we were operating upon globally. If you want to get a message across globally on your owned assets, you need to do that in the same way across the world.
back to top 

The Center for Innovative Technology (CIT) announced this week the release of the Commonwealth Research Commercialization Fund (CRCF) annual report for FY2014, showing growth in new patents, products and innovative companies.
Karen Jackson, Virginia’s Secretary of Technology, said, “CRCF plays an important role in the acceleration of innovation in the Commonwealth by funding essential research and commercialization projects. The investments we make in research commercialization plant the seeds that are growing the New Virginia Economy.”
back to top 

Bioinformatics giant Illumina (NASDAQ: ILMN) is getting into the accelerator game, along with other players in the life sciences and other fields. On Wednesday it announced the first three startups chosen to start the program this fall at its San Francisco lab space.
San Diego-based Illumina, which makes genomic analysis systems, unveiled the program in February, joining the growing list of academic institutions, venture-backed groups, and life science corporations building start-up space in the Bay Area and beyond. Not least among the benefits of the Illumina accelerator program is access to the company’s high-end gene sequencing systems, which are installed in the lab space Illumina has leased near the Mission Bay campus of the University of California, San Francisco.
back to top 

The Eastern Shore Entrepreneurship Center (ESEC), Rural Maryland Council (RMC), and Maryland Capital Entreprises (MCE) have joined together to offer the third annual Eastern Shore Business Competition. All three organizations strive to advance and expand the entrepreneurial ecosystem on Maryland’s Eastern Shore, as well as throughout Maryland, and collaborate to execute a business competition that will draw greater attention to the Eastern Shore and attract entrepreneurs to the opportunities and resources available in the region. The application process for the competition has begun and we are looking forward to your submissions!
The purpose of the competition is to promote and encourage the startup of new businesses and in turn the creation of new jobs on Maryland’s Eastern Shore.
back to top 

Ideas are the juice that powers our economy with innovation happening fast on multiple technology fronts. Rapid developments are in play in areas as diverse as 3D printing, Ultra HD, sensors, health care, automotive electronics, agriculture, transportation, biotech and genetic mapping.
The $211 billion consumer electronics (CE) industry is at the vanguard of innovation. Just last year, the U.S. Patent Office issued a record 277,835 patents. We are at the beginning of a surge of technology advances that we will all benefit from.
back to top 

On a massive bus in the heart of America, a group of tech entrepreneurs and investors are spreading glad tidings about entrepreneurship and startups in cities that don’t exactly scream innovation. Many people have tried to jumpstart the innovation engine across the country, but few have as much experience and clout to pull it off as Steve Case, co-founder of AOL, and chairman of DC-based investment firm Revolution.
back to top 

Chairs: we sit in them, work in them, shop in them, eat in them and date in them. Americans sit for most of their waking hours, 13 hours every day on average. Yet chairs are lethal.
This grim conclusion may surprise you, but 18 studies reported during the past 16 years, covering 800,000 people overall, back it up. In 2010, for example, the journal Circulation published an investigation following 8,800 adults for seven years.
back to top 

The National Institutes of Health (NIH) has launched a pilot program to help life science entrepreneurs commercialize their technology, based on a course developed by the University of California, San Francisco. The course was first taught last fall by the Entrepreneurship Center at UCSF and Steve Blank, architect of the Lean LaunchPad framework. UCSF and Blank adapted the Lean LaunchPad methodology to be applicable for life science and healthcare ventures.
back to top 
Antibiotic resistance is taking its toll on the pharmaceutical industry: Drugs are getting retired from clinical circulation, because many are starting to get rendered ineffective, according to an article from Washington University of St. Louis. It highlights the work of WUSTL’s Michael Kinch, associated vice chancellor of its Center for Research Innovation in Business:
back to top 

University of Maryland, Baltimore’s $200 million proton therapy center will begin treating cancer patients in a year.
The center’s first of five treatment rooms will begin treating patients in October 2015. The entire building is expected to be complete in two years. The 110,000-square-foot building is part of the University of Maryland BioPark in Baltimore off Martin Luther King Boulevard.
back to top 

The Center for the Advancement of Science in Space (CASIS) has announced grant awards for three projects focused on enabling technologies from the International Space Station (ISS). These awards stem from the CASIS Request for Proposals (RFP) “Enabling Technology to Support Science in Space for Life on Earth.” CASIS is the nonprofit organization managing research onboard the ISS U.S. National Laboratory.
The purpose of this RFP was to identify and support technology development projects that would enable increased use of ISS for Earth benefits—for example, improvements in hardware/capabilities or methods to improve bandwidth, throughput, or quality of future research projects.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 22
American Tap Room

October 23
Universities at Shady Grove

October 28
British Embassy

November 5- 6
FLCVirtual

November 6
IBBR
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|
 Dutch biotech MIMETAS B.V. announces that it has raised 5.2 million dollar to fund its expansion as a global leader in organ-on-a-chip technology. Venture capital investors Zeeuws Investerings Fonds (ZIF) and Participatiemaatschapij Oost Nederland (PPM Oost) have joined forces with national and regional partners to fund development and sales of MIMETAS’ unique 3D disease- and tissue modelling technologies. Dutch biotech MIMETAS B.V. announces that it has raised 5.2 million dollar to fund its expansion as a global leader in organ-on-a-chip technology. Venture capital investors Zeeuws Investerings Fonds (ZIF) and Participatiemaatschapij Oost Nederland (PPM Oost) have joined forces with national and regional partners to fund development and sales of MIMETAS’ unique 3D disease- and tissue modelling technologies.
Jos Joore, co-founder and Chief Business Officer of MIMETAS, acknowledges that these resources arrive at a strategically important moment: “Collaborations with top-tier pharmaceutical companies are expanding rapidly, an example of this is the recently announced 1.6 million dollar kidney-on-a-chip project with Roche, Pfizer and GlaxoSmithKline. The funds will be used to scale up the production of OrganoPlates™ and strengthen our activities in predictive preclinical model development, thus consolidating our leadership in this highly competitive field.”
back to top 
 Wednesday, October 15, 2014, 08:30am – 11:45am Wednesday, October 15, 2014, 08:30am – 11:45am
Please join BioHealth Innovation and the National Institutes of Health in a session to learn more about the Small Business Innovation Research (SBIR) program, one of the largest sources of early-stage capital for innovative small companies in the United States. SBIRs are non-dilutive federal research and development grants and contracts that fund innovative research efforts intended for commercialization by small business
This free event brought to you by BioHealth Innovation allows you to hear directly from the largest NIH Institutes on what they are looking for in a high quality grant application. These Institutes include the National Cancer Institute (NCI), National Heart Lung & Blood Institute (NHLBI), National Institute of Allergy & Infectious Disease (NIAID), and the National Institute of Neurological Disorders & Stroke (NINDS).
After remarks from each Institute, we will break out into a speed networking session to talk about your project with program managers.
Contact Ethan Byler at ebyler@biohealthinnovation.org if you are interested in scheduling one-on-one meetings.
back to top 

Don’t miss the third annual full-day Innovation 2 Commercialization (I2C) conference featuring three informative plenary sessions to help launch, commercialize and fund YOUR technology to make your business venture profitable!
Hear panels on innovation, commercialization and financing featuring speakers from MedImmune, Brain Sentry, Naval Research Lab and more. Plus, learn about the “Amplimmune Story” and enjoy lunch as you discuss issues affecting your innovation or small business with a subject matter expert from a federal lab, university tech transfer office, venture capital firm, business service organization and others who serve as your table host.
back to top 

The patent covers novel MDCK cells that have been adapted to grow in serum-free culture medium as well as cultivation techniques MedImmune uses to increase vaccine production titres.
According to the patent the modified cells support replication of attenuated influenza virus to a log10 TCID50/mL of at least 7, which is a significant advance on previous highest viral titres – around 4 log10 TCID50/mL – described in the literature .
back to top 

Joel S. Marcus, founder, chairman, and chief executive of Alexandria Real Estate Equities, has focused the real estate investment trust on developing properties for the life sciences industry. While it operates in other biotechnology and medical technology hubs from New York to San Francisco, the Pasadena, Calif., company’s largest cluster of properties is in Greater Boston, where it owns 3.5 million square feet of space in Cambridge’s Kendall Square, along Route 128, and in Boston’s Longwood Medical Area. On a visit to Cambridge, the 67-year-old Marcus spoke with Globe reporter Robert Weisman. Here’s what he found out:
back to top 

As Alexandria Real Estate Equities (NYSE: ARE) marks the 20th anniversary of its founding, the provider of state-of-the-art real estate for the science and technology industries is seeing occupancy, demand and new development all reach record levels.
“It’s an interesting time because we’re seeing the innovation economy doing miraculously well,” said Joel Marcus, founder and CEO of Pasadena, Calif.-based Alexandria.
back to top 

MedImmune, the global biologics research and development arm of Anglo-Swedish drug major AstraZeneca,…
In order to access this content you need to be logged into the site and have an active subscription or trial subscription. Please login, take a free trial or subscribe in order to continue reading.
back to top 

Swiss drug major Roche (ROG: SIX) has acquired exclusive rights to a primer extension-based target enrichment technology and associated patent applications filled by therapeutic target discovery company AbVitro.
AbVitro and Roche scientists are to collaborate on the development and application of the technology, which will be used to support next-generation sequencing directly from blood or other biological samples, a key advantage for clinical sequencing applications. It will be incorporated into Roche’s sequencing unit research and development pipeline to support the strategy of providing a full next-generation sequencing workflow solution for clinical sequencing.
back to top 

Join the BioBuzz networking group at our next free event. Along with 100’s of the region’s bioscience workers, you too could be making new connections, getting jobs and helping to build a stronger, more connected industry through the BioBuzz community!
The event is always free and we offer free drinks to the first 50 to 100 who arrive depending on how much we’ve been sponsored on a given month. People from all around the the region are coming out each month for this unique and welcoming monthly happy hour. It’s a great place to meet up with coworkers past and present, make new connections or just catch up on the latest industry gossip If you’re new and haven’t yet made it out to an event, then we hope that you’ll join us this month and see what all the Buzz is all about.
back to top 

A Johns Hopkins neuroscientist hopes to fuel future research projects by turning his expertise into a video game that stars a dolphin.
Hopkins neuroscientist John Krakauer, who studies movement and human obsession with movement, worked with Hopkins software architects Omar Ahmad and Promit Roy, and Baltimore artist Kat McNally to create the game “I Am Dolphin.” The game was released through iTunes Oct. 9 and costs $2.99.
back to top 

The University Senate made progress on the policies and guidelines that address non-tenure-track faculty — or professional track faculty, as they will soon be addressed, after a proposal passed in the senate yesterday.
The proposal, which passed by a vote of 61–12 with four abstentions, aims to create an overall title for faculty who are not on the tenure track but contribute to the university through teaching, research or service.
back to top 

The National Institutes of Health on Thursday awarded almost $32-million in grants to more than two dozen institutions to devise innovative ways of helping researchers handle huge sets of data seen as increasingly central to future medical discoveries.
The grants are the first outlay in a project, announced last year and known as Big Data to Knowledge, that’s expected to involve more than $600-million in spending by 2020. Its goals include developing and distributing methods, software, and tools for sharing, analyzing, managing, and integrating data into medical research.
back to top 

In 2014, startups took home nearly $1M in cash and prizes from the InvestMaryland Challenge. For 2015 we have new categories and are racking up partner prizes like cash grants, incubator space, legal advice and consulting services.
Start your application today! Applications are due December 12th.
$100,000 Grand Prize Categories
- IT HARDWARE/SOFTWARE
Enterprise Software, Data Analytics, Social Media & Apps, E-Commerce and Marketing/ADTECH.
- DEFENSE & SECURITY
Cybersecurity, Unmanned Systems, Defense, Communications Infrastructure, Public Infrastructure and Grid Security.
- LIFE SCIENCES
Biopharmaceuticals, Medical Devices & Diagnostics, Nutraceuticals, Agriculture Bio & Aquaculture and Healthcare IT.
- SUSTAINABILTY & EXPLORATION
EdTech, Energy, Solar Power, Space & Satellite Technologies, Climate Change/Weather, Water Management and Materials Science.
back to top 

Nominations are now being accepted for the 2015 FLC Awards. Since its inception in 1984 the FLC Awards have become one of the most coveted honors in the technology transfer field, with over 200 Federal laboratories honored for their work in projects that advance the mission of technology transfer. To reflect the diversity in scope and number of technology transfer efforts undertaken by federal laboratories and their partners, seven categories of awards will be presented:
back to top 

A California-based business accelerator that considers applicants based on their test scores is considering launching a Baltimore chapter.
Founder Institute already operates in 61 cities, and now it’s looking for its first Baltimore class. Local organizers will be hosting events throughout the month of October to determine whether any local entrepreneurs meet their standards – talented folks who would be a part of the four-month program that would start later this year or toward the beginning of 2015.
back to top 

A business accelerator that admits people based on their entrepreneurial potential — not just their business idea — is looking for its first Baltimore class.
Founder Institute, a California-based organization that operates accelerator program in 61 cities, is considering launching a Baltimore chapter. Local organizers will be holding engagement events in October to gauge interest and determine if any local entrepreneurs are good candidates. The four-month program could start later this year or early next.
back to top 

The ETC (Emerging Technology Centers) www.etcbaltimore.com,Baltimore City’s award-winning technology innovation centers, announced today that The Abell Foundation will continue its support of AccelerateBaltimore™for 2015. Six companies will be selected for this intensive 13- week program and will be awarded $25,000 each to help propel their business ideas forward.
“We are absolutely thrilled that The Abell Foundation is continuing its support of AccelerateBaltimore (AB),” said Deborah Tillett, ETC’s president. “Of the 16 companies to successfully complete the program, 81% are still in business and have raised over $2.5 million in follow-on funding.”
back to top 

A pilot program launched this week in four NIH institutes looks to speed up development and commercialization of new products and services generated by projects funded through the agency’s Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) awards.
The first class of 21 three-person teams of researchers and entrepreneurs gathered in Chevy Chase, MD, this week, concluding Wednesday, for the launch of the NIH Innovation Corps (I-Corps™) Team Training Pilot Program. The teams are all based at therapeutics, diagnostics, and medical devices companies funded with NIH SBIR Phase I grants, awarded to establish feasibility of proof of concept for commercializable technology.
back to top 

For more than 3,000 years, traditional Chinese medicine was the predominant medical treatment in China. In recent decades, the practice has gained popularity in the the west.
Herbal medicines and various mind and body practices, such as acupuncture and tai chi have become a fashionable way to treat or prevent health problems.
An ancient practice finds its place in modern society. This is the Beijing Chinese Medicine clinic in Santa Monica, Los Angeles.
back to top 

T, business, human capital and other stakeholders can now use cloud-based analytics to more effectively attract, acquire, serve and engage customers, Deloitte Digital announced today. Deloitte Digital has created an analytics offering for Wave, the Salesforce Analytics Cloud that is designed to offer greater flexibility than on-premises solutions. Deloitte Digital’s approach to cloud-based analytics is designed to help companies create strategies for success in a new environment. An example of Deloitte Digital’s commitment to Salesforce Analytics Cloud is its Member Connect solution, a health care industry-focused accelerator with an intuitive approach to the customer journey focusing on omni-channel interactions and a holistic view of the customer.
back to top 
Of the 115 healthcare accelerators in the world, 87 are in the U.S., most of them are geared to digital health and are under two years old. Although many accelerator companies have created jobs and there have been some exits, most have yet to show anything for the investment in them. That’s understandable given the age of the average accelerator and given that it can take seven years before a company can realize a return on capital for investors. But the consensus of a new report published by the California HealthCare Foundation is that we should expect some consolidation soon.
back to top 

Downtown Partnership of Baltimore Inc. is launching a grant program aimed at getting more tech firms to consider moving downtown.
The nonprofit is offering six $10,000 grants to tech firms willing to give office space a try downtown for at least a year. Firms must locate to within Downtown Partnership’s “management authority area,” a 106-block area that includes the central business district, part of midtown’s west side and the west side of downtown up to Greene Street.
back to top 

While all The Seven Habits of Highly Effective People listed by Stephen Covey remain relevant and timeless, the one which resonates with me most is the seventh — sharpen the saw. In the life sciences industry, Covey’s concept of continuous improvement is more than just a habit, but a way of life. This is especially true for those who work in pharma and biopharma manufacturing — striving to maintain high quality, be on time with delivery, increase productivity (often with fewer resources) and so on. If you work in manufacturing, you are probably tempted to stop reading and get back to work.
back to top 

Health information technology (HIT) refers to a broad spectrum of technologies, ranging from personal health-monitoring applications to big data analytics. The venture capital firm Rock Health recently reported that venture capital funding in the HIT field reached $3 billion for 2014, well surpassing the $1.9 billion invested in the sector during 2013.[1] The LSN research team tracks investors in early stage life sciences, and we have noticed a growing interest in HIT as well.
back to top 

Mayor Vincent C. Gray, Interim Deputy Mayor for Planning and Economic Development M. Jeffrey Miller and DC Innovates today announced grants for eight tech startups through the Digital DC Tech Fund (DDCTF). DDCTF is a catalytic fund that provides grants of $25,000 to $200,000 along with a customized mentorship program for early- and growth-stage technology ventures in the District of Columbia.
“Each of these grantees represents a bright future for technology, innovation and economic growth in the District of Columbia,” Mayor Gray said. “Through the Digital DC Tech Fund, my administration is able to provide resources and opportunities that will allow grant recipients to grow their companies and continue to make the District a place where innovative companies can start, develop and thrive.”
back to top 

For creative writing, Joyce Carol Oates got it right when she advised, “Be daring, take on anything.”
But when you are trying to make a good first impression on you future boss, concision and confidence sets a qualified applicant apart from one who doesn’t sound sure of her own experience.
back to top 

Andrew Laver has long worked with life science startups through his work as an angel investor, venture capitalist and investment banking. Now he’s getting that perspective from one of Healthbox’s newest accelerators in Salt Lake City.
As I mentioned in another post, it’s interesting that an accelerator would take on so many medical device companies (four). But as Laver points out, it goes with the territory because there are so many in the region. Salt Lake City has the biggest concentration of medical device manufacturers in the country, according to Utah’s economic development corporation. It has more than 100 medical device companies.
back to top 

Failure to diagnose the Ebola case quickly in Texas raises questions about electronic health records, known as EHRs.
Information entered by a nurse was not transferred to doctors in an emergency room. With more of our medical records going digital, failures in the relatively new technology will become more important to all of us.
back to top 
|
|
|



|
In This Issue
|
|
About BHI
BioHealth Innovation (BHI) is a regionally-oriented, private-public partnership functioning as an innovation intermediary focused on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Maryland.
|
|
|
|

October 15
Johns Hopkins University – Montgomery County

October 23
Universities at Shady Grove

October 28
British Embassy

November 5- 6
FLCVirtual

November 6
IBBR
|
BioHealth Job Opportunities
|
Newsletter designed and distributed by:

|
|
|
 |
|
The information contained in this website and newsletters is for general information purposes only. The information is provided by BioHealth Innovation via its newsletters, but not written or endorsed in any way by BioHealth Innovation unless otherwise noted. While we endeavor to keep the information up to date and correct, we make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.
|
|
|
|
|
|
|

BioHealth Innovation, Inc. (BHI), a regional private-public partnership focusing on commercializing market-relevant biohealth innovations and increasing access to early-stage funding in Central Maryland, announced today that venture capitalist, Tania Fernandez, Ph.D., has joined the BHI team as a strategic advisor. Dr. Fernandez will be a member of the management team for a new BioHealth Gap Fund, which will provide up to $50 million in seed and early-stage equity investments to therapeutics, medical device, diagnostics, and health IT companies in Maryland. Additional BioHealth Gap Fund management team members include Richard Bendis, Ram Aiyar, Todd Chappell, and Ken Malone, who each bring domain knowledge and industry access to the fund.
“Dr. Fernandez has ten years of experience as a venture capitalist in the life sciences/biotechnology industry. Her work in Silicon Valley, along with her research experience at the National Cancer Institute, makes her a tremendous asset to the BHI team,” said Richard Bendis, BHI President & CEO. “Dr. Fernandez brings a West Coast investment perspective, and she will have an active role in helping to manage the BioHealth Gap Fund. She will also support our BHI Entrepreneurs-in-Residence and clients: helping our startups to grow and raise strategic funding.”
back to top 

The next generation of the nation’s only anthrax vaccine will be made in Lansing, an executive for Emergent BioSolutions Inc. said Tuesday.
There won’t be any new jobs as a direct result of the $29 million federal contract Emergent received earlier this month for production of the new drug, Adam Havey, president of the company’s biodefense division, said. But the new version of the vaccine will help keep current employees working.
back to top 

For the majority of drugs, end-of-lifecycle planning usually involves a reining-in of marketing costs. But Sucampo CEO Peter Greenleaf is doing the exact opposite. In the firm’s second-quarter earnings call, he announced the company is “doubling-down” on Amitiza, the constipation treatment which has been on the market for eight years and which will soon face the threat of generic competition.
Amitiza owns just 1% of the overall constipation market, which includes a number of OTC options, a representative for the drugmaker told MM&M. Sucampo co-markets Amitiza with Takeda.
back to top 

Medical equipment supplier Becton Dickinson & Co (BDX.N) has agreed to buy CareFusion Corp (CFN.N), a maker of infusion pumps and other medical devices, for $12.2 billion in cash and stock, marking the latest multibillion-dollar healthcare sector deal.
Becton said on Sunday it would pay a total of $58.00 a share – $49.00 in cash and 0.0777 of a share of Becton Dickinson – for each share of CareFusion, representing a premium of 26 percent to the closing price on Oct. 3.
back to top 

Pipeline updates are highly awaited events in the pharma/biotech sector as they play an important role in deciding whether or not to invest in a particular company. These updates provide information on experimental drugs and at times give an insight into the commercial potential of the candidate once it is successfully developed and commercialized.
Roche (RHHBY – Analyst Report) specializes in cancer drugs. Immunotherapy has received a lot of attention in recent times as pharmaceuticals majors focus their research and development efforts on the same.
back to top 

GlaxoSmithKline has just launched a $5 million Innovation Challenge Fund to advance open-access technology in the bioelectronics space.
The funding’s aimed toward biz-savvy academics and startups that are working to create a new class of treatments that aren’t necessarily pills or injections, but rather are mini implantable devices. GSK says:”The hope is that these devices could be programmed to read and correct the electrical signals that pass along the nerves of the body, to treat disorders as diverse as inflammatory bowel disease, arthritis, asthma, hypertension and diabetes.”
back to top 

University of Maryland, Baltimore County, and University of Maryland, College Park will work with MITRE Corp. on a new cyber security research and development center in Montgomery County.
McLean, Va.-based MITRE was selected by the National Institute of Standards and Technology for a $29 million award to operate a new federally funded research and development center dedicated to cyber security. The center will be housed at the National Cybersecurity Center of Excellence in Montgomery County.
back to top 

Tasly Pharmaceuticals, Inc. made its official debut at the Natural Products Expo East 2014, held from September 17-20, 2014 in Baltimore, MD. As the leading East Cost trade show in the natural, healthy and organic products industry, attracting 22,000 professionals and representing 33% of the natural products industry, Expo East 2014 was a perfect opportunity for Tasly to introduce Deepure, its inaugural line of nutraceuticals. The line includes three condition-specific, whole-food and herb-based formulas, namely, ProHeart PLUS, ImmunoPower PLUS, and Re-Memory PLUS. All Deepure nutraceuticals are gluten-free and made without chemicals, preservatives, artificial colors, flavors, sweeteners, or gelatin. Deepure will be available in stores nationwide later this Fall.
Tasly also participated in two very attractive marketing and sponsorship opportunities located in New Products Pavilion, including Best of East Press Showcase and New Products Showcase.
back to top 

To attend a NICE/UKTI Scientific Advice Seminar for developers of devices and diagnostics.
Learn how to prepare for an evaluation by NICE, the UK’s health technology assessment body.
The seminar will cover:
- “Value” from the perspective of NICE
- How value propositions link to the need for specific evidence
- The types of evidence considered by NICE
- The general principles of health technology assessment (HTA)
- Advantages of doing business in the UK
Who should attend?
- Developers of medical technologies, in particular SMEs
- Investors in the field of medical technologies
- Anyone new to the world of health technology assessment
- Academics, scientists, or clinicians who want to know more about how NICE assesses the value of innovative products
The seminar program is funded by UKTI to allow you free event registration (standard event rate – $550)
Read More
| | | | | | | | | | | | | |