 ROCKVILLE, Md. and SUZHOU, China, Jan. 23, 2025 (GLOBE NEWSWIRE) — Ascentage Pharma (Nasdaq: AAPG) (HKEX: 6855) announced today the pricing of its U.S. initial public offering of 7,325,000 American depositary shares (“ADSs”), at a public offering price of $17.25 per ADS, before underwriting discounts and commissions. Each ADS represents four ordinary shares of Ascentage Pharma. The gross proceeds from the offering, before deducting underwriting discounts and commissions and other offering expenses payable by Ascentage Pharma, are expected to be approximately $126.4 million. In addition, Ascentage Pharma has granted the underwriters a 30-day option to purchase up to an additional 1,098,750 ADSs at the initial public offering price, less underwriting discounts and commissions. The ADSs are expected to begin trading on the Nasdaq Global Market on January 24, 2025, under the ticker “AAPGV” on a “when-issued” basis, and on January 27, 2025, under the ticker symbol “AAPG” for “regular-way” trading. The offering is expected to close on January 28, 2025, subject to customary closing conditions.
ROCKVILLE, Md. and SUZHOU, China, Jan. 23, 2025 (GLOBE NEWSWIRE) — Ascentage Pharma (Nasdaq: AAPG) (HKEX: 6855) announced today the pricing of its U.S. initial public offering of 7,325,000 American depositary shares (“ADSs”), at a public offering price of $17.25 per ADS, before underwriting discounts and commissions. Each ADS represents four ordinary shares of Ascentage Pharma. The gross proceeds from the offering, before deducting underwriting discounts and commissions and other offering expenses payable by Ascentage Pharma, are expected to be approximately $126.4 million. In addition, Ascentage Pharma has granted the underwriters a 30-day option to purchase up to an additional 1,098,750 ADSs at the initial public offering price, less underwriting discounts and commissions. The ADSs are expected to begin trading on the Nasdaq Global Market on January 24, 2025, under the ticker “AAPGV” on a “when-issued” basis, and on January 27, 2025, under the ticker symbol “AAPG” for “regular-way” trading. The offering is expected to close on January 28, 2025, subject to customary closing conditions.

 BALTIMORE, Jan. 24, 2025 /PRNewswire/ — In two separate procedures, MedStar Health cardiac experts at MedStar Union Memorial Hospital reached new heights in cardiovascular care when they performed the state’s and the system’s first transcatheter tricuspid valve replacements (TTVR) using a recently FDA-approved valve, to treat life-threatening tricuspid regurgitation. On Jan. 7th, the male and female patients, both in their 80’s, received the revolutionary valves delivered via a catheter, foregoing the need for conventional open-heart surgery.
BALTIMORE, Jan. 24, 2025 /PRNewswire/ — In two separate procedures, MedStar Health cardiac experts at MedStar Union Memorial Hospital reached new heights in cardiovascular care when they performed the state’s and the system’s first transcatheter tricuspid valve replacements (TTVR) using a recently FDA-approved valve, to treat life-threatening tricuspid regurgitation. On Jan. 7th, the male and female patients, both in their 80’s, received the revolutionary valves delivered via a catheter, foregoing the need for conventional open-heart surgery.


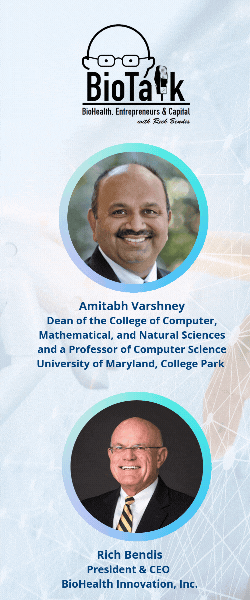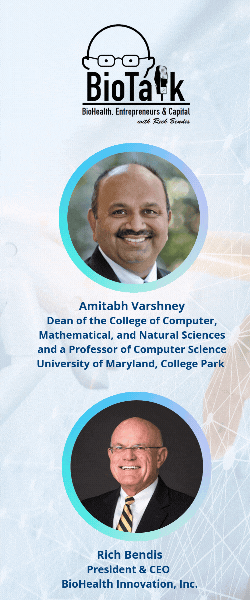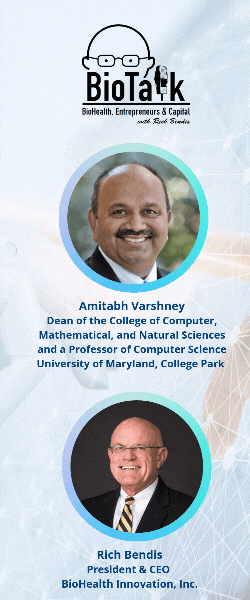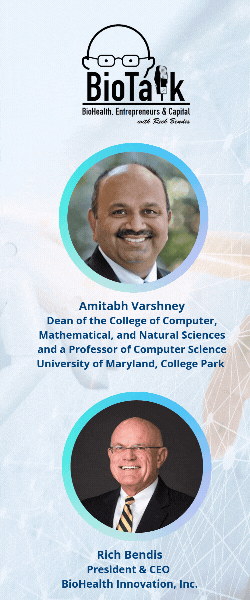 In this episode of BioTalk, host Rich Bendis is joined by Amitabh Varshney, Dean of the College of Computer, Mathematical, and Natural Sciences and a Professor of Computer Science at the University of Maryland, College Park. Together, they explore the groundbreaking advancements in artificial intelligence and health computing driven by the University of Maryland (UMD).
In this episode of BioTalk, host Rich Bendis is joined by Amitabh Varshney, Dean of the College of Computer, Mathematical, and Natural Sciences and a Professor of Computer Science at the University of Maryland, College Park. Together, they explore the groundbreaking advancements in artificial intelligence and health computing driven by the University of Maryland (UMD).





