BioHealth News Archive
Protenus cofounder Robert Lord is returning to JHU medical school, joining LionBird Ventures as partner – Technical.ly Baltimore
When Robert Lord cofounded Protenus with Nick Culbertson in 2014, it required choosing to put medical school on hold. After six years and plenty…
Read MoreEngineered Medical Systems and The LaunchPort™ Partner with Longeviti Neuro Solutions
Engineered Medical Systems, Longeviti Neuro Solutions and The LaunchPort™ announced today that they have entered into an agreement to place new, low temperature, low…
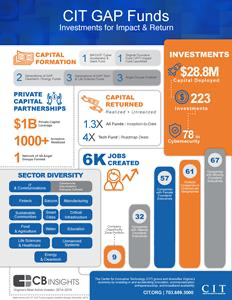
Read MoreCIT GAP Funds 2019 Impact Report Showcases $28.8 Million in Capital Deployed to Date
Herndon, VA, Jan. 14, 2020 (GLOBE NEWSWIRE) -- The Center for Innovative Technology (CIT) today released the 2019 CIT GAP Funds Impact Report, detailing…
Read MoreTritower Financial sells Gaithersburg biotech buildings to Alexandria Real Estate Equities for $53.25M – Washington Business Journal
The seller converted the office space — leased heavily by local biotech companies — to lab suites, then sold the buildings just three years…
Read MoreNew Decade, Old Challenge: BioManufacturing Workforce Development Remains Key to Industry Growth · BioBuzz
The BioHealth Capital Region (BHCR) experienced significant change in 2019 that has set the table for an intriguing 2020. How 2020 plays out across…
Read MoreEmerging Technology Centers Baltimore lands on top 10 incubators list – Baltimore Business Journal
A Baltimore business incubator has been named among the best in the world. Emerging Technology Centers in Baltimore has been recognized by UBI Global…
Read MoreNew Medical Device That is Proving Effective Against the Worlds Toughest Viruses, Including: Ebola, Hep C, HIV, West Nile and Smallpox Wins FDA Designation “Breakthrough Device”
LOS ANGELES, Jan. 15, 2020 /PRNewswire/ -- USA News Group – A new study published in the journal Molecular Therapy reports that researchers found a…
Read MoreJPM: Roche, Illumina unveil 15-year cancer diagnostic tie-up | FierceBiotech
SAN FRANCISCO—Roche has inked a 15-year partnership with Illumina in oncology, which will include collaborating on new companion diagnostic indications for the DNA sequencing…
Read MorePrecigen Receives FDA Orphan Drug Designation for PRGN-3006 UltraCAR-T™ in Patients With Acute Myeloid Leukemia (AML) – BioHealth Capital Region
GERMANTOWN, Md., Jan. 6, 2020 /PRNewswire/ — Precigen, Inc., a biopharmaceutical company specializing in the development of innovative gene and cellular therapies to improve the lives…
Read MoreNIST wants to update iEdison tech-transfer portal to get it out of the ’90s
The National Institute of Standards and Technology is seeking public feedback on its plan to revamp the Interagency Edison (iEdison) System, an online portal where…
Read MoreMaryland Department of Commerce names Allyson Redpath entrepreneurship director – Bizwomen
The Maryland Department of Commerce has created a new role focused on the state's startup ecosystem and tapped a former investment banker and angel…
Read MoreBiobuzz Directory – Local Biotech, Hememics Biotechnologies Inc., has secured a $2.5 million investment
Rockville, Maryland-based Hememics Biotechnologies Inc. has secured a $2.5 million investment from AMVI Partners, an international investment firm with offices in McLean, Virginia. The startup is working…
Read MoreIBT Vaccines Receives $3.9 mil. to Advance Development of IBT-V02, the First Multivalent Toxoid Vaccine for MRSA | Integrated BioTherapeutics
IBT Vaccines, a wholly owned subsidiary of Integrated Biotherapeutics Inc., announces it has received $3.9 million to advance the development of the IBT-V02 vaccine for…
Read MoreThink universities are making lots of money from inventions? Think again. – The Washington Post
When Daria Mochly-Rosen discovered a compound in her lab that promised to lessen the effects of heart attacks, she set out to convince pharmaceutical…
Read MoreNotes and Takeaways from JPM 2020
The big deal about the just-completed J.P. Morgan (JPM) 38th Healthcare Conference had little to do with big M&A deals, because there weren’t any.…
Read More