BioHealth News Archive
When working remotely, constant communication is key to more action – Washington Business Journal
Not long ago, working from home was an option for some of us. Today, it’s the norm for nearly all of us. Unless your…
Read MoreGSK, AstraZeneca in talks for joint U.K. COVID-19 diagnostics project: Bloomberg | FierceBiotech
GlaxoSmithKline and AstraZeneca are considering forming a joint laboratory to help the U.K. government stretch and expand its supplies for COVID-19 diagnostic tests, according…
Read MoreBHI and DLA Piper Host Webinar on Navigating the CARES Act for Life Sciences Companies in the BioHealth Capital Region
On April 3rd, 2020, DLA Piper and BioHealth Innovation, Inc. (BHI) presented an overview of the key provisions for BioHealth Capital Region Life Sciences…
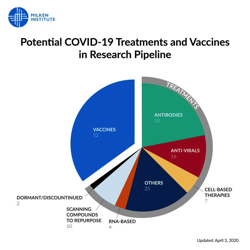
Read MorePotential COVID-19 Treatments and Vaccines in Research Pipeline
We're currently tracking 95 potential treatments and 52 potential vaccines in the pipeline to combat hashtag#COVID19. There are thousands of researchers all over the…
Read MoreCoronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients of NIH Funding | grants.nih.gov
The NIH is deeply concerned for the health and safety of people involved in NIH research, and about the effects on the biomedical enterprise…
Read MorevenBio Closes $394 Million Life Sciences Venture Capital Fund | BioSpace
SAN FRANCISCO--(BUSINESS WIRE)-- venBio Partners LLC today announced the closing of venBio Global Strategic Fund III (“venBio Fund III”), its third life sciences venture…
Read MoreThree Long-Time Emmes Employees Are Named VPs
ROCKVILLE, Md., April 6, 2020 /PRNewswire/ -- Emmes today announced that Heather Hill, Dr. Adam Mendizabal and Dr. Nilay Shah have been promoted to…
Read MoreYou’ve Never Heard of BARDA, the Agency That May Rescue You From Coronavirus
One of America’s biggest companies has teamed up with one of the country’s least-known federal agencies to make doses of a coronavirus vaccine. Lots…
Read MoreWashington Business Journal’s Sara Gilgore to anchor DC Inno
A few weeks ago, I was in New Orleans for a jam-packed wedding weekend, complete with a crawfish boil, second line parade, Hawaii-themed 30th birthday…
Read MoreEmergent BioSolutions gets $14.5M in federal funding to expedite COVID-19 plasma therapy development | TechCrunch
Last week, we spoke to the head of Emergent BioSolutions’ Therapeutics Business Unit Dr. Laura Saward about her company’s work developing plasma-based potential treatments…
Read MoreJohns Hopkins gets FDA approval to test blood plasma therapy to treat COVID-19 patients | Hub
The U.S. Food and Drug Administration approved a clinical trial Friday that will allow Johns Hopkins University researchers to test a therapy for COVID-19…
Read MoreAccelerator Series / Maryland SBDC
COVID-19 Notice for Maryland SBDC Clients, Stakeholders, and Partners We are committed to serving our clients during the COVID-19 (Coronavirus) health emergency. Consulting Services…
Read MoreEmergent Announces Partnership with the U.S. Government
We're proud to announce a partnership with the U.S. Government on a comprehensive response to expedite development of our plasma-derived therapy to address hashtag#COVID19.…
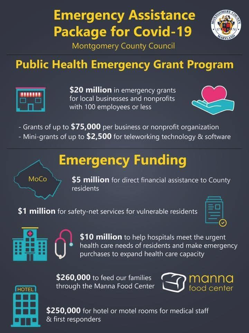
Read MoreEmergency Assistance Package for Covid-19
Information regarding the hashtag#montgomerycountymd Public Health Emergency Grant Program passed by County Council yesterday. The County Executive's staff is preparing regulations and plans to…
Read MoreSolicitations | SBIR.gov
Solicitations play an important role in making the general public aware of funding opportunities available to the small businesses of the nation. Participating Federal…
Read More