BioHealth News Archive
Venture Building: Why the Old Way of Venture Capital Is Dead – Grit Daily News
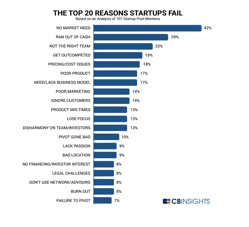
In 2020, the failure rate of startups was around 90%. Research showed that 21.5% of startups failed in the first year, 30% in the…
Read MoreGeoffrey Lynn, co-founder Avedia, SVP, Synthetic Immunotherapies, Vaccitech, joins BioTalk
Geoffrey Lynn, SVP, Synthetic Immunotherapies, Vaccitech, visits Rich Bendis to discuss his career from NIH, to CEO of Avidea, to their merger with Vaccitech.…
Read MoreNEXT GENERATION TB TEST DEVELOPED WITH ELLUME TECHNOLOGY RECEIVES APPROVAL FROM KEY GLOBAL REVIEW PANEL FOR USE IN MORE THAN 100 LOW-RESOURCE, HIGH BURDEN COUNTRIES
FREDERICK, Md. and BRISBANE, Australia, Jan. 10, 2022 /PRNewswire/ -- Digital diagnostics company Ellume today announced that the QIAreach QuantiFERON-TB test (QIAreach QFT), developed with…
Read MoreHow Johns Hopkins Inventors’ Vision for Early Cancer Detection Got a $2.1B Boost – Johns Hopkins Technology Ventures
Johns Hopkins researchers Nickolas Papadopoulos, Ken Kinzler and Bert Vogelstein have spent their careers working on ways not just to treat cancer but to…
Read More2 technologies of which hospitals should be wary
Blockchain and "connected health" technology are two trends hospitals should not rush to adopt, according to two hospital innovation executives. Discussions of blockchain have…
Read MoreArcellx Announces Pricing of Initial Public Offering – Arcellx
GAITHERSBURG, Md., Feb. 04, 2022 (GLOBE NEWSWIRE) -- Arcellx, Inc. (NASDAQ: ACLX), a biotechnology company reimagining cell therapy through the development of innovative immunotherapies…
Read MoreBaltimore Fishbowl | University of Maryland, Baltimore County reaches nation’s highest level as research university –
The University of Maryland, Baltimore County has received a Carnegie Classification, the nation’s highest designation for research universities. Two other universities in Maryland –…
Read MoreAmalgam named Best SaaS-Enabled Digital Health Platform!
Global Health & Pharma recently presented their seventh annual Biotechnology Awards and named Amalgam the Best SaaS-Enabled Digital Health Platform. The Global Health &…
Read MoreAmerican Gene Technologies’ HIV Clinical Trial Shows Blood Markers of Efficacy in Two More Patients
ROCKVILLE, MD. (PRWEB) FEBRUARY 02, 2022 Data from a Total of Five Patients Demonstrates Critical Markers of the Company's HIV Cure Gene & Cell…
Read MoreEmmes Supports New Collaboration to Accelerate COVID-19 Research for Children
Emmes, a global, full-service Clinical Research Organization dedicated to supporting the advancement of public health and biopharmaceutical innovation, today announced its role as a…
Read MoreKey lawmaker: ARPA-H won’t be part of NIH – STAT
WASHINGTON — A new research agency aimed at developing breakthrough medical technologies won’t be housed within the National Institutes of Health, a key lawmaker…
Read MoreFresh Off Best Year Ever, Lab Giant Alexandria To Venture Into New Territory
The leading developer of life sciences properties rode the industry's wave last year, recording 4.1M SF of leases signed between October and December, more than double its…
Read MoreUSP and WHO Renew Official Relations to Continue Strengthening the Global Medicines Supply Chain | LinkedIn
On Saturday, January 29, the World Health Organization (WHO) Executive Board approved the renewal of official relations with the United States Pharmacopeial Convention (USP)…
Read MoreThe Clues to the Next Variant Surge Are All Around Us
When scientists in South Africa noticed an uptick in Covid-19 cases in the Gauteng Province last November, they began investigating the source. These researchers…
Read More2021 Global life sciences sector outlook | Deloitte
Navigating the pandemic has been an all-encompassing, once-in-a-lifetime challenge. Globally, life sciences companies responded with leadership and are emerging stronger. How will life sciences…
Read More