|

The latest chapter in Human Genome Sciences’ battle to fend off a hostile takeover bid by British pharma giant GlaxoSmithKline played out in a Rockville courtroom Thursday morning.
A Montgomery County Circuit Court judge shot down an HGS shareholder’s request for a temporary restraining order to invalidate the “poison pill” the Rockville biotech enacted last month to make it a less attractive acquisition target.
back to top 

Johns Hopkins has had many milestones since it first opened its hospital in Baltimore in 1889. It pioneered the acceptance of women to medical school and the use of rubber gloves in surgery, discovered restriction enzymes and the brain’s natural opiates, birthed multiple medical specialties including neurosurgery and pediatrics, and developed life-saving procedures such as renal dialysis, CPR, and the “blue baby” operation that paved the way for modern heart surgery. May marked another major milestone for the nation’s best hospital for 21 years in a row: the opening of its brand new high-tech clinic.
We had the opportunity to sit down with The Johns Hopkins Hospital and Health System’s President, Ronald R. Peterson, to discuss their new clinical building. Peterson has an impressive and storied background at Johns Hopkins, which is why he’s ideally positioned to talk about the milestone.
back to top 

If you are an emerging advanced technology, life sciences or professional services company or a foreign business looking for a soft landing in the U.S. market, the Business Innovation Network of Montgomery County, Maryland has the perfect place for you. The Innovation Network business incubators are located throughout Montgomery County adjacent to Washington, D.C. with its talented workforce and strategic access to the federal and commercial marketplace, all in a sophisticated, diverse community. The Network was founded by the Montgomery County Department of Economic Development in 1999 with the opening of the Shady Grove Innovation Center and specializes in helping young companies realize their potential. Since its inception the Business Innovation Network has worked with over 250 teams of entrepreneurs and graduated about 100 companies. Over the last 10 years the Network has grown to five business incubation centers that offer the critical combination of highly flexible, modern office and lab space and business support services.
back to top 

MedImmune in Cambridge UK is reaching out to academics and biotech companies in a bid to improve the industry’s poor neuroscience track record.
Together with AstraZeneca in Boston, Massachusetts, MedImmune – the global biologics unit of AstraZeneca – is setting up a collaborative unit at its Granta Park HQ with the aim of producing drugs to treat neurodegenerative conditions, long term pain and neuropsychiatric conditions.
Iain Chessell, vice-president R & D Neuroscience said: “There have been no new approvals of completely novel mechanisms for treating pain for at least a decade – if not more – and current treatment only works in a third to half of patients.
back to top 
 Below is an editorial suggesting the nation could become more economically competitive by helping remove barriers to connect our federal lab technology, human and physical resources to the private sector. Without question, Maryland has the most to gain from this national initiative. We are home to the nation’s largest concentration of federal laboratories and many federal lab researchers live in Maryland. To its credit, the state has launched new programs to support commercialization and partnering among the state’s considerable academic research and development assets. Since federal labs are creatures of federal legislation, these efforts need to extend to federal labs, augmented with federal policy reforms. Now is the time for the state to lead the Maryland Congressional delegation, working with other state congressional delegations, to work on a bi-partisan basis to enact pathways for better connecting the human, physical and technology assets of our federal labs with their regions. Below is an editorial suggesting the nation could become more economically competitive by helping remove barriers to connect our federal lab technology, human and physical resources to the private sector. Without question, Maryland has the most to gain from this national initiative. We are home to the nation’s largest concentration of federal laboratories and many federal lab researchers live in Maryland. To its credit, the state has launched new programs to support commercialization and partnering among the state’s considerable academic research and development assets. Since federal labs are creatures of federal legislation, these efforts need to extend to federal labs, augmented with federal policy reforms. Now is the time for the state to lead the Maryland Congressional delegation, working with other state congressional delegations, to work on a bi-partisan basis to enact pathways for better connecting the human, physical and technology assets of our federal labs with their regions.
back to top 

Thursday, June 14, 2012 from 10:00 AM to 12:00 PM (ET)
Rockville, MD
A free and open forum to:
- Discuss InvestMaryland implementation progress and investment strategy
- Detail state venture funding resources to seed and early-stage companies
- Address questions from the business community
back to top 

Laurie Boyer, president of the Maryland Economic Development Association, is the new executive director of Rockville Economic Development Inc.
Boyer has more than 15 years of government and economic development experience, according to a statement from REDI.
She served more than five years as director of the Frederick County Office of Economic Development and earned her certified economic developer designation from the International Economic Development Council in 2006.
back to top 

The State of Maryland's Pulse on the Bio Industry.
back to top 

It's been almost a decade since the Human Genome Project was completed, yet despite the best efforts of thousands of scientists around the world, hopes for cures for a wide range of diseases remain unfulfilled.
Last fall, a remarkable group of leaders came together to find new ways of overcoming the barriers that have prevented more progress in medical research. A report from the Milken Institute, released today, Accelerating Innovation in the Bioscience Revolution, recaps the discussions from that gathering – the 2011 Milken Institute Lake Tahoe Retreat.
back to top 

Bernard T. “Bernie” Ferrari, an accomplished corporate strategist and management consultant to Fortune 50 companies, has been named the next dean of The Johns Hopkins University’s Carey Business School.
Ferrari, whose appointment is effective July 1, is the second dean to lead the Carey Business School since it was established in 2007. He succeeds Yash P. Gupta who stepped down last June.
back to top 

Private Sector Entrepreneur-in-Residence Program in partnership with the NIH/OTT Monday, June 11, 12:00 pm to 12:30pm ET
Presenters: Richard Bendis Founding President and CEO Innovation America and Mark L. Rohrbaugh, Ph.D., J.D. Director Office of Technology Transfer National Institutes of Health Department of Health and Human Services
BioHealth Innovation, Inc.'s (BHI) Entrepreneur-in-Residence (EIR) program is designed to be an active partner with research institutions to source, fund, and grow high-potential, early-stage products through project-focused companies. The entrepreneurs in the program support the formation of new companies based upon innovative discoveries in the areas of drugs, vaccines, therapeutics, diagnostics, and medical devices from the intramural research programs at the NIH and Food and Drug Administration (FDA), as well as from universities and businesses. The EIR will find, evaluate, and support the development of new start-up companies based upon technology license agreements from technology transfer offices or equivalent units within the research institutions.
back to top 

United States Senator Robert Menendez (D-NJ) has introduced legislation that will revive the Therapeutic Discovery Project Tax Credit, which funneled $1 billion in tax breaks and grants to biotech companies across America in 2010. The program impacted about 3,000 small US companies that year. “Biotech labs employ dedicated scientists and researchers, whose discoveries could lead to a ground-breaking cures for cancer, diabetes, Alzheimer’s, or HIV/AIDS,” Menendez said in a statement released last week. “Manufacturing these breakthrough therapies is already creating thousands of high-paying jobs, and extending this critical tax credit will not only create more good jobs here in America, but keep us at the forefront of life-saving innovation.”
back to top 

Universities have historically been on the front lines of translating innovative research into novel medicines and technologies useful to patients. With that in mind, the 2012 BIO International Convention will look to highlight the role of academia in the advancement of the biotechnology field through the BIO Academic Park and the Translational Research Forum. Hosted by the Biotechnology Industry Organization (BIO), this year's global event for biotechnology will take place June 18-21, 2012 at the Boston Convention and Exhibition Center in Boston, MA.
"The BIO Academic Park will give Convention attendees the opportunity to connect and start conversations that could lead to partnerships, and most importantly, establish a tighter link between academic, industry representatives and investors," said Dr. Abigail Barrow, Founding Director of the Massachusetts Technology Transfer Center and Program Co-Chair of the 2012 BIO International Convention.
back to top 
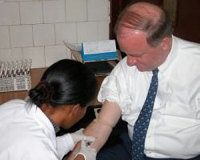
Johns Hopkins researchers say it’s going to be a hard sell to get physicians to stop screening healthy men routinely for prostate cancer with PSA testing, despite recommendations from the U.S. Preventive Services Task Force (USPSTF) that the cancer screening does more harm than good.
Patient expectations, malpractice fears cited The researchers surveyed physicians, 74.4% of whom said patients expect PSA testing.
back to top 

The pharmaceutical industry rightly calls the stage in drug development between basic research and clinical trials the “Valley of Death.” This is when a potential treatment that’s worked in mice, monkeys, and the like catapults to a phase 1 clinical trial to assess safety. It’s rare.
Francis Collins, MD, PhD, director of the National Institutes of Health, calls this period “where projects go to die.” The reason: $.
Matthew Herper writes in Forbes that the cost of developing a new drug is $4-11 billion, not the $1 billion that Pharma often claims. Yet even that $1 billion is unimaginable, especially when you put a face on a rare disease and witness what the family goes through to leap to phase 1.
back to top 

Eli Lilly is participating in a new investment fund which will focus primarily on early-stage drug development opportunities in Canada as a whole and Quebec in particular.
The fund, which will be operated by investment investment group TVM Capital, will have an initial size of $150 million. As well as Lilly, other backers include Teralys Capital (which is putting in $65 million), BDC Venture Capital, Fondaction and Advantus Capital Management.
back to top 

Accelerator, the venture-backed biotech startup machine, has made its name over the past decade as a hotspot for financing life sciences companies in Seattle with big dreams and potential. Now it’s considering expanding its model for starting biotech companies in other life science clusters around the world, including New York.
Plans are still in the exploratory stage, but the idea is that Accelerator would remain headquartered in Seattle and build a network of satellite labs in four or five other locations around the world, says Carl Weissman, the co-founder and CEO of Accelerator. Accelerator’s existing venture backers, and some potential new investors, have expressed interest in a more far-reaching version of Accelerator, Weissman says.
back to top 
S an Francisco-based digital health incubator Rock Health revealed the six startups that will take part in its “pop up” incubator in Boston this summer. The Boston program is a bit different from those that took place at Rock Health headquarters in California: Those were five-month long spring and fall programs and the Boston program’s duration will be just under three months. The startups will present at a demo day on August 24, 2012. Harvard Medical School will play host to Rock Health’s third class and the institution’s community of clinicians and industry experts will work with the startups to refine their strategy. an Francisco-based digital health incubator Rock Health revealed the six startups that will take part in its “pop up” incubator in Boston this summer. The Boston program is a bit different from those that took place at Rock Health headquarters in California: Those were five-month long spring and fall programs and the Boston program’s duration will be just under three months. The startups will present at a demo day on August 24, 2012. Harvard Medical School will play host to Rock Health’s third class and the institution’s community of clinicians and industry experts will work with the startups to refine their strategy.
Here’s how Rock Health describes the six startups (we had only heard of one previously) in its first Boston class:
back to top 

BIO International Convention organizers are hoping for the usual surge in attendance provided by the Boston biotech community at the event scheduled for June 18-21 at the Boston Convention and Exhibition Center. While it may not reach the 22,000 level achieved at the last Boston BIO in 2007, it should surpass the 15,600 who came to last year's event in Washington, D.C. Exhibitors will occupy more than 215,000 sq. ft of exhibit space.
Attendance for the event has been off its historic highs since the Atlanta event in 2009 which drew about 14,000. Organizers attribute the drop that year to the H1N1 influenza epidemic which surfaced a few weeks before the convention and to the economic downturn. Attendance has been slowly recovering since 2009.
back to top 
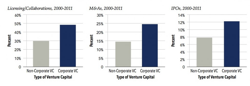
Pharma corporate venture was back in the biotech news today with the release of Burrill & Company’s June 2012 report. An interesting article by Vinay Singh evaluated the impact of Pharma corporate venture capital (CVC) investing, and the key takeaway is that CVC-backed companies have a higher rate of overall success than those without their involvement.
While a similar takeway has been published before by Windhover’s StartUp about a year ago, these data suggests a fairly robust effect from a large dataset. The analysis includes 2907 therapeutics companies that raised venture capital dollars between 2000-2010 across 5100 rounds of financing. Corporate VCs were investors about 10% of companies, and this pool of 286 companies had what appears to be a markedly higher hit rate: a ~60% higher rate of licensing deals, M&As and IPOs.
back to top 

Smartphone adoption among physicians has started to level but there’s been an “explosion” of adoption in tablets, Manhattan Research’s VP of Research Monique Levy said during a recent webinar. Levy said that Manhattan’s survey of physicians in the US found that 62 percent now have some kind of tablet device, almost twice as many as last year.
“I still cannot believe some of this data. I had to double and triple check it because it is just astounding,” Levy said. The majority of these tablet toting doctors are iPad owners, but even among that group Levy was surprised to find that even they are planning to buy an additional tablet device in the next few months.
back to top 

Only a few companies have ever been successful enough to call themselves Big Biotechs. If boards and shareholders lack vision and guts, we’ll look back in few years and wonder why the Big Biotechs went extinct.
The group of Big Biotechs includes companies like Amgen, Gilead Sciences, Biogen Idec, and Celgene. They grew from scrappy venture-backed startups with a dream into big, independent, profitable, diversified enterprises. They have enduring ability to create new jobs and new medicines. They are like ballasts in a stormy industry.
back to top 

Following on the heels of two hospitals, the University of Notre Dame has become the first university to strike a collaboration with Cleveland Clinic aimed at commercializing medical innovations from its faculty and researchers.
Through the collaboration, Cleveland Clinic Innovations will essentially do for Notre Dame what it does for the Clinic — help turn employee ideas into marketable products that generate financial returns for the organization.
back to top 
|