
This invitation-only event is free for executive level biotech leaders and is presented by BioHealth Innovation, VirginiaBio, Paragon Bioservices, Inova, University System of Maryland and MedImmune/AstraZeneca. Maryland, Virginia, and Washington, DC set the bar high for biotech innovation. So please join us for our Annual BioHealth Capital Region Forum that will highlight the accomplishments of today and chart our successes of tomorrow.
We are excited to bring you an exceptional line up of speakers. The program will feature sessions that focus on Advancing Science and Accelerating Innovation.

The University System of Maryland announced this month the launch of its Maryland Momentum Fund, which will provide $25 million to support startups formed within the system.
The university system has already committed $10 million to the initiative, and is working alongside the University of Maryland, Baltimore County as well as UM Ventures — a joint research and innovation program between this university and the University of Maryland, Baltimore — to obtain the remaining $15 million from local venture capitalists and angel investors, said Julie Lenzer, UM Ventures co-director.

The National Cancer Institute has picked the Johns Hopkins Montgomery County Campus as the site for a new laboratory building for its epidemiology and genetics researchers.
The planned 70,000-square-foot building will bring together about 134 scientists and employees now working in separate facilities in Gaithersburg and Frederick, about 25 miles apart.

Patients everywhere take their medication with emocha.
This World TB Day, we want to say thank you to our partners around the world who are uniting to #EndTB.
Patients across three continents are using the emocha platform to facilitate TB treatment, and we’re proud to support hundreds of healthcare workers as they fight to eradicate TB.
Public health departments in California, Maryland, Texas, Missouri, Washington, Rhode Island, and Puerto Rico have adopted emocha’s video DOT platform for active and latent TB monitoring. Sites in Australia, India, and Mexico have also implemented the technology, proving that video DOT is an effective strategy for combating global TB.
On Tuesday, March 7, 2017, the University of Maryland (UMD) Robert E. Fischell Institute for Biomedical Devices and RTI International (link is external) welcomed nearly 150 representatives of Maryland’s medical device ecosystem to a daylong event focused on bringing products from invention to market in the state. The event, Medical Device Innovation in Maryland: from Technology to Lab to Market, took place at the UMD campus in College Park, and culminated with a tour of the soon-to-be new home of the A. James Clark School of Engineering: A. James Clark Hall.
“This is the start of an incredibly exciting time for biomedical devices in Maryland – and you’re here at the beginning,” University of Maryland Provost Mary Ann Rankin told attendees.

Millions of bodies suffer a loss of soft tissue — namely skin or muscle — each year. It can result from trauma, natural aging, or even surgery, like a mastectomy for breast cancer patients. Typically, the only option to repair such damage is surgery, and transferring tissue from one part of the body to another.

Symbiomix today announced that the US Food and Drug Administration (FDA) has accepted for filing the Company’s New Drug Application (NDA) for its lead investigational product candidate Solosec™ (secnidazole oral granules), an innovative antibiotic designed to treat bacterial vaginosis (BV). In accordance with the FDA’s priority 6-month review designation, the agency has established a user-fee goal date under the Prescription Drug User Fee Act (PDUFA) of September 17, 2017. Symbiomix submitted the NDA filing in January 2017.
Solosec™ is a potent, next-generation, investigational 5-nitroimidazole antibiotic with enhanced pharmacokinetic properties, which will be the first and only single-dose oral therapy approved for BV if approved by the FDA. The FDA previously designated Solosec™ as a Qualified Infectious Disease Product (QIDP) for the treatment of BV and granted it Fast Track designation, which made Solosec™ eligible for priority review and at least 10 years of market exclusivity.

The co-founders of a Johns Hopkins-backed startup had personal motivations for developing better health care tracking technology.
The mother of Sathya Elumala, who is CEO of Multisensor Diagnostics, suffers from multiple chronic health conditions and spends time in hospitals almost every month. Gene Fridman, the company's chief technology officer, lost a sister who had a chronic condition that wasn't detected soon enough.

The University of Maryland University College is tearing itself apart -- on purpose.
The university, which gets nearly all of its funding from tuition revenue, has long relied on enrolling large numbers of students associated with the military for financial stability. After teaching students at military bases across Asia and Europe for decades, the university was quick to realize the potential of the internet and became a pioneer in the field of distance education.

May 18, 2017 - 8 a.m. - 4 p.m. - Hilton Baltimore
Join Governor Hogan for the first Maryland Governor’s Business Summit. Explore topics on human capital, global trends and business strategy. Connect with a wide array of Maryland’s business leaders.

Newsflash: The Association of University Technology Managers met in Hollywood, Fla., and had its own Final Four last week.
All of the finalists of the conference’s pitch competition had a common bond: They each had ties to Maryland universities such as Johns Hopkins and University of Maryland. All four also received funding from TEDCO’s Maryland Innovation Initiative.

Across the commonwealth, families are feeling squeezed by stagnant wages and rising costs. Too many Virginians are working part-time instead of full-time; too many college graduates are working in jobs that don’t require a four-year degree.
Virginia’s anemic economic growth rates have trailed the national average for five straight years. We even fell to the bottom ten states for economic growth in 2014. More people are moving out of Virginia than moving in. Our tax climate ranking fell to 33rd in 2017. We’ve dropped out of the top 10 on the CNBC list of Best States for Business, after being number one just five years ago.

GlaxoSmithKline and Regeneron have given their backing to a new project that will use patient data held by the UK Biobank.
The firms will get free access to the samples in return for covering the cost of sequencing it and, after a period of exclusivity, they will then allow the data to be plugged back into the resource for wider use.

Eli Lilly and Company has publicized plans to invest $850 million in the United States throughout 2017.
The media release, delivered Friday, follows a meeting between Lilly executives, federal, state and local government officials at Lilly Technology Center in Indianapolis, Indiana.

Once again, more than three-quarters of U.S. venture capital (VC) dollars went to companies in California, New York and Massachusetts in 2016, according to data from the PricewaterhouseCoopers (PwC)/CB Insights’ Moneytree Report Explorer. Approximately 53.3 percent of all VC capital went to California companies, down nearly 4.4 percent from the states peak in 2014 (57.7) and down 3.9 percent from 2016. While California’s share declined, both Massachusetts and New York saw increases in their share of VC dollars invested:

Get a sneak peak of the Mobile eXploration Lab (MXLab) – the country’s largest, most advanced mobile STEM laboratory! MdBio is launching the MXLab for the 2017/2018 school year, and we want to give teachers across the state the first chance to see the lab in action and provide feedback on our activities for Maryland high schools.
Join us at one of five events across the state to get a sneak peek of the MXLab plus a hands-on teacher professional development activity for your classroom.

What’s next for the pharmaceuticals industry, amid digital disruption and rapid technological advances? McKinsey’s Martin Dewhurst sat down for a wide-ranging conversation with David Epstein of venture firm Flagship Pioneering to explore ways the sector could shift in coming years. In this interview, part of our Biopharma Frontiers series on how the pharmaceutical industry is evolving and how leaders can adapt, Epstein discusses the next horizon of innovation, critical elements of an effective business, and the kind of culture he tries to foster. An edited transcript of their conversation follows.

Host: UM BioPark & Wexford + Technology
Location: UM BioPark Life Sciences Conference Center, 801 W. Baltimore Street, Baltimore, Maryland 21201
Date: March 30, 2017 - 5:00pm to 6:30pm
Please join the BioPark's Monthly Networking Event along with collegues from the Maryland Proton Treatment Center. Mingle with MPTC leadership and staff, BioPark tenants, and UMB faculty and staff.

Vaccines may be available for pneumococcal infections, but none of them, including the widely used Prevnar 13, can cover all strains. SutroVax is one of the startups trying to solve that problem, and today raised $64 million to test its approach in humans.
New investors Frazier Healthcare Partners and Pivotal bioVenture Partners led the Series B financing round for Foster City, CA-based SutroVax. Also participating in the round were earlier investors Abingworth, Longitude Capital, Roche Venture Fund, and CTI Life Sciences Fund. In addition to the $60 million Series B investment, SutroVax says the earlier investors are also putting another $4 million into the company.

Pulmocide raised $30.4 million in a Series B round of financing to support the early clinical development of its lead inhaled drugs against respiratory syncytial virus (RSV) and pulmonary aspergillosis. The fundraising round was led by new investor SR One and included the Longwood Fund and existing investors SV Life Sciences, F-Prime Capital, Johnson & Johnson Innovation–JJDC, and Touchstone Innovations.
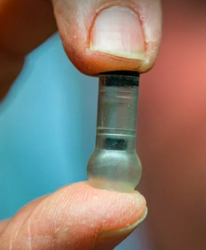
Patients could one day self-administer vaccines using a needleless, pill-sized technology that jet-releases a stream of vaccine inside the mouth, according to a proof-of-concept study conducted at UC Berkeley.
The study did not test vaccine delivery in people, but demonstrated that the technology, called MucoJet, is capable of delivering vaccine-sized molecules to immune cells in the mouths of animals. The technology is a step toward improved oral vaccine delivery, which holds the promise of building immunity in the mouth’s buccal region of cells, where many infections enter the body. When patients hold the MucoJet against the inside of their cheek, the device releases a jet stream that directly targets the buccal region. This region is rich in immune cells but underutilized in immunology because of the challenge of efficiently penetrating the thick mucosal layer in this part of the oral cavity with existing technologies, such as the oral spray often used for influenza vaccination.

Dear Friends of CONNECTpreneur:
I hope everything is well! This is a "Last Call" for our April 4 Big Idea CONNECTprenuer SPRING FORUM at the Hyatt Regency in Bethesda, MD. We expect over 500 CXOs, founders, entrepreneurs, angel investors, etc., and we will be completely SOLD OUT again. So, please sign up now! We have unparralled CXO to CXO networking, plus an amazing program: Fireside chat with DAVID TRONE, Co-Founder and Owner, Total Wine & More Awesome Entrepreneur "War Stories" Panel (moderated by GLEN HELLMAN (aka Mr. Cranky), angel investor and CEO of Driven Forward) featuring:· NIGEL JONES, CEO of Koolspan and Managing Director of TWJ Capital· BETH MALONEY, President of Palladian Partners· D.P. VENKATESH, VP of Broadsoft and CEO of mPortal PLUS 9 awesome early stage companies will present their companies.

The National Science Foundation's Small Business Innovation Research (SBIR) program provides small business with equity-free funding to conduct research and development (R&D) work and de-risk technology for commercial success. The NSF SBIR program accepts innovative proposals that show promise of commercial and societal impact in almost all areas of technology.
SBIR Phase I proposals are expected to undertake R&D with the aim of establishing technical feasibility or proof of concept. Successful applicants will receive a grant of up to $225,000 over a period of 6 to 12 months (the period to be decided by the company).
Successful SBIR proposers will receive funding about 6 months after the solicitation deadline. Companies that receive a Phase I award are eligible to apply for a Phase II award (award amount up to $750,000; duration 2 years).

In order for America to maintain its innovative and technological competitive advantage, it is imperative that current policy design favor high growth formation through the catalytic means of institutional financing. The Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programs have historically served as crucial financing to help de-risk early-stage technology development and enable validation that encourages follow-on private institution capitalization. With new trends in the Internet of Things, artificial intelligence, advanced materials, genomics, environmental sustainability, renewable energy, national security, and many other areas evolving over the years ahead, national governments bear the responsibility for helping seed the inventive fire that enables economic growth as we move forward.

WEDNESDAY, APRIL 19, 2017, 8:30 AM - 12 PM | WASHINGTON, DC
If you do business with the government, you are a target for cyber-attacks!
If you are a government contractor, join us to learn how to implement a strong and cost effective cybersecurity program that will strengthen your company's cybersecurity posture and help you meet requirements necessary to do business with the federal government. As an attendee, you can receive two customized assessments to help identify your company’s level of exposure: (1) a mini cyber-risk review from CyberRx that will assess your company’s cyber capabilities within the Cybersecurity Framework; and (2) a threat intelligence assessment from Wapack Labs that can help to identify if your organization has been or will be the victim of an attack. Space is limited. Register today.

Friday, April 14, 2017 7:30 AM - 9:30 AM
Bethesda Marriott - 5151 Pooks Hill Road Bethesda, MD 20814
With the transition to the Trump administration, change is coming rapidly in the form of legislative proposals, regulations (or repeal thereof), and policy statements. How will all this affect the ability of tech companies to raise the capital they need to grow. Amid this shifting ground, we’ve asked an expert panel of our own Maryland technology companies to talk about their recent successes and challenges in raising capital and their outlook for the ability of Maryland technology companies to do the same. Our panel, moderated by David Straut of Wells Fargo, includes two long-time local tech CFOs who have had recent success raising money and the Director of the Maryland Venture Fund, which oversees investments in approximately 50 local technology ventures
Financial Executives Forum: FEF is devoted to peer-to-peer connections and programs that highlight the latest topics of interest for the region’s leading business and financial executives. FEF encourages participants to connect as well as learn from some of the area’s preeminent executives, policymakers and thought leaders.

Please join the Maryland Women's Business Center for an Open House at our Rockville office.
Learn about our program and expanded services
Celebrate and meet MWBC clients who are growing their businesses
Meet our business counselors from Montgomery, Frederick and Prince George's Counties
Network with local government officials, women entrepreneurs, sponsors, partners, and the local business community.
And, if you've been thinking about entering our 2017 StartRight! Women's Business Plan Competition, it's a great opportunity to ask past participants and winners about the process.
Light refreshments will be provided by MWBC clients.
There is no cost to attend, but RSVPs are requested. Click the register button below.
For questions or more information, contact Donna Gallagher, donna@marylandwbc.org, 301-315-8091.

Wednesday, March 29, 2017, 02:00pm - 04:00pm
USDA ARS and the USDA SBIR program formed a partnership to encourage USDA SBIR applicants to develop research collaborations with ARS scientists and/or to license ARS technologies. The purpose is to increase the likelihood of success by providing both money and technologies to small U.S. businesses. The relevant language in the SBIR’s “Request for Application” states: "Additional factors that will be considered in the review process include whether an application involves a Cooperative Research and Development Agreement (CRADA) with a USDA laboratory, or a license to a USDA technology, or is a resubmission.”
This webinar will include an overview of the USDA SBIR program and crafting a competitive SBIR proposal. Also an overview of ARS and how to develop a successful research collaboration (CRADA) with an ARS scientist and/or license an ARS technology will be discussed. The webinar will address coordinating the statement of works in an ARS CRADA and a USDA SBIR proposal.

March 31st, 2:30-4:30pm
Join us to learn more about bioscience as an industry and career opportunities available in Montgomery County
Presentation on BioScience + Tour of Montgomery College BioLab for staff and partners working with job seekers
To RSVP, email Heather Henry at hhenry@worksourcemontgomery.com