
Rockville-based Sucampo Pharmaceuticals Inc. finalized its acquisition of the Japanese biotech R-Tech Ueno for $275 million, officials said Monday.
In August, Sucampo (NASDAQ: SCMP) made a $278 million all-cash offer to acquire R-Tech Ueno, a Japanese pharmaceutical company that manufactures Amitiza, Sucampo's flagship drug that eases drug-related constipation using fatty acid-derived compounds known as prostones.

Care Progress, LLC announced today that it was awarded a Phase II Small Business Innovation Research (“SBIR”) grant from the National Science Foundation (“NSF”). The Phase II award will fund the further development of the company’s CarePrompter© software platform, which improves outcomes for cancer patients as well as workflow and profitability for oncology departments and practices.
“It provides support for further development of our critical technology. With this award, we will accelerate our plans to develop additional functionality for cancer providers and patients within a large and growing market.” Tweet this "This Phase II SBIR award is a significant milestone for us," said Howard Isenstein, Founder of Care Progress. "It provides support for further development of our critical technology. With this award, we will accelerate our plans to develop additional functionality for cancer providers and patients within a large and growing market."

With payers increasingly rewarding value and expectations of the customer experience on the rise among all stakeholders, one of the key ways pharmas will differentiate their products is through understanding their customers’ pain points and providing tools and services that address them. For today’s physician, EHRs are a real pain, and prior authorizations are another. So we were interested to learn of a Avhana Health, a startup that is currently piloting a cloud-based clinical decision support system that sits on top of four of the big EHR systems – Epic, Allscripts, AthenaHealth and Greenway.

Supports K@W's Innovation Content Pfizer Inc. raked in more than $12.4 billion in revenue in both 2007 and 2008 from just one drug, Lipitor. The cholesterol-lowering mega-blockbuster accounted for more than a quarter of the pharmaceutical giant’s total revenue in those years.
But those golden days couldn’t last.

DATE: Wednesday, December 9, 2015LOCATION: Montgomery County Department of Economic Development, 111 Rockville Pike, Suite 800, Rockville, MD 20850
The FDA Technology Transfer program seeks to advance the development and commercialization of inventions that result from regulatory science research and development conducted at the FDA. Regulatory Science can be defined as the science of developing new tools and technologies to assess the safety, efficacy, quality, and performance of FDA-regulated products. Technology Transfer tools used by the FDA Technology Transfer Program are the same tools used by Federal Agencies and FDA technology transfer-related policies are similar to the policies of the NIH and CDC. However, the range of technologies developed at the FDA is more diverse- a reflection of the FDA’s role in ensuring the safety of food, drugs, biologics, medical devices, tobacco, and veterinary medicines.

The Maryland Technology Development Corp. (TEDCO) is bringing in an industry veteran to turn its $106 million Maryland Venture Fund into a regional leader in venture funding.
Andy Jones, an entrepreneur and venture capitalist, joined TEDCO on Monday as managing director of the Maryland Venture Fund. Jones’ prime task will be to elevate the fund from a supporting investor to a leader in venture capital that drives more money to local companies.

PharmaCyte Biotech, Inc. (PMCB), a clinical stage biotechnology company focused on developing targeted treatments for cancer and diabetes using its signature live-cell encapsulation technology, Cell-in-a-Box®, announced today that, upon the recommendation of the European Medicines Agency (EMA), the European Commission has granted the Orphan Drug designation to PharmaCyte’s subsidiary, PharmaCyte Biotech Europe Limited, for PharmaCyte’s pancreatic cancer treatment. Receiving Orphan Drug designation for PharmaCyte’s pancreatic cancer treatment carries with it 10 years of marketing exclusivity in countries in the European Union. In addition, the EMA provides special assistance in the development of PharmaCyte’s treatment for pancreatic cancer.
The Orphan Drug designation in the European Union is given to drugs for life-threatening diseases with low prevalence, or that make it unlikely an investment in a drug to treat a life-threatening disease would be cost justified, and that demonstrate there is a significant benefit to patients being treated with the drug.

Arlington cybersecurity start-up ThreatConnect said Tuesday that it has raised $16 million from investors, led by the corporate venture capital arm of SAP’s North American subsidiary in Rockville. The next morning just down the road in Sterling, Va., a similarly-named start-up called ThreatQuotient said it raised $10.2 million, led by prolific technology investor New Enterprise Associates. A few weeks ago Arlington-based Trustar announced a $2 million in seed funding.

The D.C.-based venture capital firm Revolution Growth is bringing on additional talent.
The firm, founded by AOL co-founder Steve Case, Monumental Sports & Entertainment's CEO and owner Ted Leonsis, and Donn Davis, announced the hiring of four new vice presidents Tuesday to help spur additional investments and push for a greater return on its portfolio.

Thursday, December 10, 2015 from 8:00 AM to 10:00 AM, bwtech@UMBC
Senator Mikulski is retiring.
Governor Hogan is just getting started.
Who’s the next champion of Maryland’s life sciences sector?
Please join We Work For Health (WWFH) Maryland and the University of Maryland Baltimore County (UMBC) as we explore how to develop Maryland’s next generation of elected leaders to foster medical innovation and grow our regional bio-economy.
Over breakfast, members of the biotechnology and pharmaceutical industry, government, labor, and academic community will discuss the importance of identifying and cultivating medical innovation leaders to help address our challenges and make the most of our
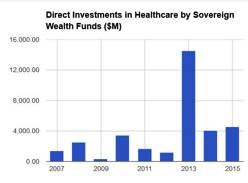
In the past couple of years there has been a rise in sovereign wealth funds’ investments in healthcare. These cover a wide range of areas from hospital chains to pharmaceutical companies, but it also includes direct investments in digital health startups. China Broadband Capital invested in smartphone diagnostic developer Scanadu and French Groupe Arnault includes Clue, a maker of female health apps, among its investments. Alaska Permanent Fund is one of the founding investors in Denali Therapeutics, a biotech business targeting neurodegenerative disorders.

Two years ago, life science startups were only six percent of the Google Ventures portfolio.
As 2015 draws to a close, that industry now is nearly one third of Google Ventures investments and is only going to grow larger, says the president and CEO of Google's venture arm, Bill Maris.

We're another step closer to extending the life span of human beings.
Researchers in Switzerland have been studying the effect of genes on the aging process and have published their results in the journal Nature Communications. Of the 40,000 genes they studied, they found 30 that allow a variety of animals, including roundworms, zebrafish, and mice, to stay healthier and live longer. But these scientists focused specifically on genes that are also found in human beings, so the next step in their project is to see if these genes also have an impact on us as well.
Jeff Carroll inherited the DNA mutation that causes Huntington’s disease. It means that in a decade or two, he’ll lose control over his body and slowly go mad, just like his mother.
That’s the reason Carroll, 38, says he’d be in favor of gene editing embryos. He says the idea of correcting DNA errors in the next generation has no “ick factor” for him at all. “I have no compunctions about it,” says Carroll, who is a neuroscientist at Western Washington University, in Bellingham. “I am saying, please, please do mess with our DNA.”
Is Google about to take a huge dive into the health industry?
The search engine giant has just filed a patent for a “needle-free blood draw” system that could be worn on your wrist and help people who need regular draws to get blood quickly and painlessly, according to a The Verge report.