
Remedy Pharmaceuticals, a company focused on the development and commercialization of CIRARA™, a breakthrough drug for treating acute central nervous system conditions, announced that a prospective multi-center open-label pilot study of CIRARA in patients with acute traumatic cervical spinal cord injuries.

Following a $2 billion merger between DTZ and Cushman & Wakefield, David Gillece will head up the firm's newly combined offices in Baltimore.
Gillece was regional managing principal with DTZ and before that held the same position with brokerage Cassidy Turley, which combined with DTZ last year.

emocha Mobile Health, a digital health startup that’s using telemedicine as a way to confirm medication adherence, has won the support of the South Africa department of health to roll out its program for multi-drug resistant tuberculosis patients across the country following a pilot, according to a company statement.
The program, referred to as miLINC, was developed to identify, track and support these patients. For patients in primary care clinics, healthcare professionals collect critical data such as a national identification number, medical record number, mobile number, and specimen barcode. Lab results are matched against enrollment data from the clinic on emocha’s cloud-based server. Linkage Officers check results and follow up with patients. Specialized nurses check patients into specialized clinics for MDR TB.

Monday, September 21, 2015 from 8:00 AM to 12:00 PM (EDT)
This event is to gather interested small businesses seeking assistance from the Small Business Innovation Research grants program from the National Institutes of Health. This is a free event brought to you by BioHealth Innovation. Hear from the SBIR managers on current Institute funding priorities. Meet one-on-one with program managers regarding your current project. Learn of SBIR assistance provided by BioHealth Innovation.

Cerecor, a Baltimore pharmaceutical firm planning an preliminary public inventory providing, has launched a brand new medical research of an antidepressant it says will help sufferers who aren’t responding to plain remedies.
The corporate is conducting a randomized double-blind experiment to check a drug generally known as CERC-301 on 104 topics who’re experiencing extreme depressive episodes regardless of receiving different remedies. It’s a Part 2 medical trial, which suggests it’s in search of to show the drug triggers a organic response and has some effectiveness in comparison with a placebo.

In the growing age of virtual reality (VR), a startup launched by University of Maryland researchers has designed new technology to help transport your senses from wherever you are to wherever you want to be—a music concert or sporting event far away—in a way that will make you believe you are actually there.
The co-founder of VisiSonics Corporation (link is external), Professor of Computer Science Ramani Duraiswami of the University of Maryland Institute of Advanced Computer Studies (UMIACS) (link is external), explained that it all started with some key inventions in the general area of 3D sound.
University of Maryland, Baltimore County, and Johns Hopkins University are among 12 universities that will share a $20 million grant for groundbreaking nanotechnology research.
The institutions are all part of the Center for Sustainable Nanotechnology at the University of Wisconsin-Madison. The five-year, $20 million grant from the National Science Foundation will support the center research of how nanoparticles interact with live beings and the environment.

Since Congress passed the 21st Century Cures Act on July 10th, one Big Pharma company has already moved to capitalize on the legislation even before it has been passed by the Senate and become law. A good introduction to the bill can be found in this piece. In short, the bill is designed to speed up the route to market for certain therapeutics. Senate passage is estimated for around December. In the last piece I paid particular attention to one of the most consequential aspects of the Act - that it would promote the development of brand-new antibiotics. In response to the Act, there have been two interpretations of this likely influx of therapeutics.

Mark Lafferty has joined UM Ventures Baltimore as a venture associate. In this role, he provides financing and business planning advice to new companies, prepares valuations for startup companies and new products, and evaluates financial aspects and terms for licensing deals.

Synthetic Biologics, a microbiome-focused clinical-stage biotechnology company, is moving its offices to the Johns Hopkins University Montgomery County Campus. Synthetic Biologics previously had its corporate headquarters at the VisArts incubator space in downtown Rockville but is moving its staff of 15 to the JHU campus, with plans to add more staff members in the future. The company has its administrative and financial offices in Ann Arbor, Mich.
The company is developing drugs to protect the gut microbiome in an effort to maintain the natural balance of bacteria in the gastrointestinal tract, which is important to our overall health, said Kris Maly, the company’s vice president of corporate communications.

Don't miss out on this UNIQUE and FREE networking happy hour.
Meet Rockville business owners and learn more about the resources available to your business.
Some of our resource partners in attendance will be:
- BioHealth Innovation
- Rockville Economic Development, Inc.
- Maryland Women's Business Center
- Rockville Chamber of Commerce
- Maryland Economic Development Association
Please join us and take advantage of this opportunity to find out what REDI can do for you.
You will want to also be there when this year's "Rockin' in Rockville" award is presented!

According to the U.S. Chamber of Commerce, Maryland is the top state in the nation on its “Innovation and Entrepreneurship” index. Despite this strong environment, the first few years of a startup can be a financial headache.
If you are like many startups and in need of funding, or if you are looking to stretch the funding that you do have, one of the most effective strategies is to take advantage of one of the tax incentives that Maryland has created for technology companies.
University of Maryland Medicine (the University of Maryland Medical Center and the University of Maryland School of Medicine) and its Center for Metabolic Imaging and Image-Guided Therapeutics (CMIT) has begun to use MRI-guided focused ultrasound on a deep structure within the brain related to Parkinson's disease - the globus pallidus.
In the first clinical trial of its kind, researchers from the Departments of Diagnostic Radiology & Nuclear Medicine, Neurosurgery and Neurology at CMIT are using magnetic resonance imaging (MRI) to guide ultrasound waves through the intact skin and skull to the globus pallidus. The University of Maryland is one of only two sites in the United States to offer this treatment to Parkinson's patients.

Application Deadline: September 11, 2015
An Information Session Webinar about the NIH-CAP is scheduled for Tuesday, September 1, 2015 at 2:00 pm EDT (11:00 am PDT). For more information and to register for the info session, please click here. This session is open to small businesses for which HHS SBIR and STTR Phase II, Phase IIB, or Phase II portion of Fast-Track award is or was active in the past 5 fiscal years. Projects that ended before August 2010 are not eligible. Awardees from the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) can also attend.

In a message to the Johns Hopkins community today, university President Ronald J. Daniels outlined recent initiatives the university and health system are undertaking to "help bend the trajectory of our city."
As he welcomed students, faculty and staff to the start of a new academic year, Daniels recounted connections that Johns Hopkins has made with Baltimore residents through the Summer Jobs Program, school uniform drive, and ex-offenders hiring program. He also highlighted a new public school partnership, research projects, workforce training, and the upcoming launch of the HopkinsLocal economic inclusion initiative.

Roche said on Friday it had won breakthrough therapy designation from the U.S. Food and Drug Administration for an experimental haemophilia medicine, aiming for a piece of the $11 billion haemophilia drug market.
The Swiss drugmaker said its U.S.-based Genentech unit's ACE910 secured the fast-track designation as the company prepares separate Phase III trials in 2015 and 2016, the first in patients with haemophilia A with factor VIII inhibitors and the second for patients without inhibitors.

Cross border deals involving digital health startups in Israel are on the rise. In the most recent example, Johns Hopkins Technology Ventures has inked a multiyear agreement with Israeli digital health business Luminox that will set up an accelerator for Israeli digital health startups, among other things.
Luminox refers to itself as a digital health hub. It helps connect early stage companies with U.S. institutions, corporates and strategic partners.

Free, Open Entrepreneur Office Hours for University of Maryland Students, Faculty and Staff, and Regional Entrepreneurs with Bio or Tech-Based Startups or Ideas Get answers now from experienced entrepreneurs and legal/business professionals on how to build a successful startup company. Receive free and impartial advice, brainstorm business strategies, investigate funding opportunities and learn about the vast resources available to entrepreneurs.

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
Notices:
- OMB Approval for Form Changes Underway - Continue to Use Current Forms Until Further Notice
- eRA Commons Help Desk Rebranded as eRA Service Desk
Requests for Applications:
- Abuse Liability Associated with Reduced Nicotine Content Tobacco Products (R01)
- (RFA-OD-15-006)
- Office of Disease Prevention
- U.S. Food and Drug Administration, Center for Tobacco Products
- National Cancer Institute
- National Heart, Lung, and Blood Institute
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- National Institute on Drug Abuse
- Application Receipt Date(s): December 11, 2015
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.

Baltimore's tech scene will get some time in the spotlight later this month when Steve Case's "Rise of the Rest" tour rolls into town. The five-city roadshow, through which the AOL co-founder and former CEO plans to invest $1 million in early-stage technology ventures, kicks off in Baltimore on Sept. 28.
The bus tour, which is designed to bring attention and funding to startups beyond traditional tech hubs like Silicon Valley or New York City, will also give Johns Hopkins University a chance to show the part it plays in supporting Baltimore's bubbling culture of tech innovation.

Are you an entrepreneur and thinking of starting your own company? Are you a small start-up looking for local resources that will help your company succeed? Are you a small company looking to expand and enter the next stage of commercial success? If you said “Yes” to any of these questions, then this WIB event is for you!
Our panel of industry experts from MD TEDCO, BioHealth Innovation, and the Montgomery County Department of Economic Development will provide you with invaluable information about resources their organizations provide to the local business community, including idea/product development, early and late stage funding, and business accelerator programs.

ADC Therapeutics (ADCT), an oncology drug discovery and development company that specializes in the development of proprietary Antibody Drug Conjugates (ADCs) targeting major cancers, today announced that it has raised $80 million through a private placement of equity. New investors include leading European and US-based investors alongside founding investor Auven Therapeutics and participation from AstraZeneca.
The proceeds will be used to progress ADCT’s product portfolio, including ADCT-301 for lymphoma and leukemia now in Phase I and a collaboration to develop up to two ADCs for commercialisation with MedImmune, the global biologics research and development arm of AstraZeneca. ADCT’s ADCs are highly targeted drug constructs which combine monoclonal antibodies specific to surface antigens on particular tumor cells with highly potent pyrrolobenzodiazepine (PBD)-based warheads. ADCT anticipates having seven drug candidates in human clinical trials in 2017.

A lot of time, effort and money goes into the creation of new drugs. A new game Big Pharma, which came out Thursday, is giving users the opportunity to see for themselves by running a virtual pharmaceutical company.
Not unlike Big Pharma in real life, one of your biggest priorities is money. It’s up to you whether or not you want to sacrifice efficacy to make cheaper products and yield more profit.

A digital health fund to invest in Israeli digital health startups recently made its first investment — Intendu. The company provides a way for people with neurological problems stemming from traumatic brain injury to age-related cognitive decline to train the brain through a series of personalized exercises that involve body-controlled adaptive videogames. The goals are customized to each user.
Partners for digital health venture fund eHealth Ventures include Cleveland Clinic Innovations, which is providing resources to the company in exchange for equity, and one of Israel’s largest HMOs, Maccabi Healthcare, are also supporting the fund.

Google is making another move into the profitable sphere of diabetes management: Its Life Sciences arm—which will soon fall under Google's new parent company, Alphabet—is announcing a partnership with Sanofi, a French pharmaceutical company, to build new treatment products for diabetes. The two firms will collaborate on product development and new methods for monitoring the condition.
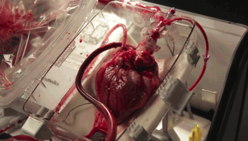
Transplant surgeons have started using a device that allows them to “reanimate” hearts from people who have recently died, and use the organs to save others.
The “heart in a box” is a wheeled cart with an oxygen supply, a sterile chamber, and tubing to clamp onto a donor heart and keep it fed with blood and nutrients. Doctors say it may extend the time a heart can last outside the body and is letting them recover hearts from donors who haven’t been eligible before.
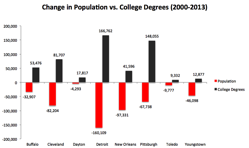
If there’s one thing that’s a nearly universal anxiety among cities, it’s brain drain, or the loss of educated residents to other places. I’ve written about this many times over the years, critiquing the way it is normally conceived.
Since brain drain seems to be a major concern in shrinking cities, I decided to take a look at the facts around brains in those places. Looking at the 28 metro areas among the 100 largest that had objective measures of shrinkage – in population and/or jobs – between 2000 and 2013, I looked what what happened to their educational attainment levels.

Planning is officially underway for the 2016 FLC national meeting scheduled for April 26-28 at the Drake Hotel in downtown Chicago. This year, a new technology 'pitch' session is under development with The Defense Innovation Initiative – Experimental (DIUx) that will feature a panel of investors who are familiar with early stage technologies, including lower Technology Readiness Level (TRL).
Pitching Ideas
All laboratories are invited to pitch their technologies to the panel of investors! The most promising ideas will be given the opportunity to receive additional support and resources to further assess and develop their concepts for commercialization through licensing, identification of potential collaboration partners, etc. Don’t miss the chance to interact with investors and gain valuable exposure of your lab’s technologies in front of an investment audience.
To submit your technologies for the ‘Pitch’ session, please download and follow the instructions on the attached flyer! For additional questions about tech submissions, contact Program Chair Kathleen McDonald at Kathleen_m@lanl.gov.
Many American cities are distinctly shaped by ties to other parts of the world. Washington has its Ethiopian community, and the restaurants that have come with it. Chicago has its Mexican neighborhoods, Minneapolis its Hmong culture, Miami its links to Cuba.
These differences — products of proximity or history or happenstance — are part of what makes New York feel culturally different from Atlanta. And they reflect the fact that New York and Atlanta look different to people living abroad.

Product development of any kind is challenging and full of unknowns, obstacles and pivots.
Medical device product development is certainly no different.
With med devices, when you throw in regulatory bodies, such as FDA and others internationally, it only complicates matters further.

D.C.-based startup incubator 1776, which filed with regulators last year to raise its first seed fund, announced Tuesday it has closed the fund at $12.5 million, half of what it had expected to raise.
1776 has backed 20 companies since launching the fund, co-investing with other groups including 500 Startups, Silicon Valley Angels and GovTech Fund. The fund typically invests an average of $100,000 in pre-Series A startups focused on highly regulated industries, such as health, education, energy and transportation.