|

Sucampo Pharmaceuticals Inc. on Thursday named Peter Kiener — whose past roles includes CEO of Zyngenia and head of biologics R&D for MedImmune — as its chief scientific officer.
Kiener, a heavyweight research hire, worked alongside Sucampo CEO Peter Greenleaf at MedImmune, where Greenleaf served as president until early 2013. Kiener then departed to launch Zyngenia Inc., a New Enterprise Associates-funded biotech focusing on antibody-based therapeutics, which he departed last year.
back to top 

Qiagen has announced the introduction of expanded functionality for its range of bioinformatics workflow solutions.
New capabilities for the Ingenuity Variant Analysis and CLC Cancer Research Workbench solutions were unveiled at the American Society of Human Genetics annual meeting, which recently concluded in San Diego.
back to top 

Tuberculosis is both tough to treat and medically cumbersome to manage. Under directly observed therapy, a medical professional has to watch a patient take his or her medication for at least six months. A Baltimore health IT startup may help lighten that load.
The Baltimore City Health Department is launching a pilot with emocha Mobile Health’s app miDOT, according to a release from the Highlandtown firm.
back to top 

The U.S. government invested $440 million in three vaccine plants in the U.S. in 2012 with the proviso that if something like a pandemic occurred, it could call on them to produce drugs that it required. With Ebola spreading, those calls have now been made.
The Ebola crisis has prompted the Biomedical Advanced Research and Development Authority (BARDA) to ask the three plant owners--Novartis ($NVS), GlaxoSmithKline ($GSK) and Emergent Biosolutions ($EBS) and their partners--to tell it what it would take for them to produce ZMapp, an experimental drug currently being produced through a novel approach using tobacco plants. BARDA wants detailed timetables and budgets for making ZMapp, Reuters reports. They are supposed to respond by Nov. 10.
back to top 

Strategic Adviser for BioHealth Innovation Inc.
Tania Fernandez came to the biotech initiative through the venture capital world, where she worked in Silicon Valley and spent 16 years managing investments in companies.
What is the biggest way your day will change in your new role? My day won't really change. I am a classified workaholic. I will continue to do what I have always loved doing: working with entrepreneurs, building companies and looking for good investment opportunities in the biotech/life sciences/health care sectors. BioHealth Innovation has built a very commendable ecosystem for startups, and I look forward to the opportunity to build out that ecosystem with them as we get ready to fund raise for the BioHealth Gap Fund.
One thing you wish everyone knew about your job? I founded DreamCatcher Ventures after having spent more than 15 years in hard-core academics and a decade in venture capital financing. I have watched people get burnt either because they were not grounded enough (which translates into operational weakness) or had their head way above the clouds (which translated to being unrealistic about long-term success and sustainability of business models). I created DreamCatcher Ventures with a simple goal to "Build the foundations for dreams." That's what I want people to know about my job. This quote by Henry David Thoreau, which is the tagline for DreamCatcher Ventures, says it all: "If you have built castles in the air, your work need not be lost; that is where they should be. Now put the foundations under them." For entrepreneurs there is a fine line between being stubborn and being persistent, an even finer liner between being a visionary and having unrealistic expectations. In my day-to-day job, I work with clients to bridge that. It is great to dream but you need to have your feet planted on the ground. I believe in the power of dreams combined with the excellence of execution.
back to top 

Colleen Worthington has been working at this university for more than 24 years. It wasn’t until this year, however, that she received her first promotion.
“No matter how much better I got or more I contributed, it was the same title from 1990 until last [semester],” Worthington said.
back to top 

Italian drugmaker Sigma-Tau is in advanced talks to sell part of its Italian operations to domestic peer Alfa Wassermann that would create an over-the-counter (OTC) powerhouse, several sources familiar with the situation said.
Sigma-Tau is working with Milan-based Four Partners, an advisory firm led by Sigma board member Guido Tugnoli, said the sources, who declined to be identified because the matter is private.
back to top 

NewLink Genetics Corp (NLNK.O) said it entered into an agreement with Roche Holding AG (ROG.VX) to develop NewLink's cancer immunotherapy, making the Ebola vaccine developer eligible to receive over $1 billion in milestone payments.
NewLink's shares jumped nearly 30 percent before the bell on Monday.
back to top 

Swiss pharmaceutical group Roche hopes to obtain fast-track US approval for a rapid diagnostic test for Ebola, its director general said in an interview published Sunday, amid the worst-ever outbreak of the killer disease.
The diagnostic test is ready for use in scientific research but the company now wants to register it for clinical use.
back to top 

Pharma giant GlaxoSmithKline (GSK) might plan an initial public offering (IPO) of a minority stake in ViiV Healthcare, a global business focused on developing treatments for HIV, reports FierceBiotech.
Created five years ago, ViiV is GSK’s majority-owned joint venture with Pfizer and Shionogi. In the past 18 months, the Food and Drug Administration (FDA) approved two new HIV treatments from ViiV: Tivicay (dolutegravir) and the combo-tablet Triumeq, which includes Tivicay. These successes come at a time when GSK is planning to slash nearly $1.6 billion from its annual budget.
back to top 

It’s no secret that Washington has a funding problem, with lawmakers and federal departments currently operating under unprecedented fiscal constraints.
It’s a very different story for the city’s entrepreneurs.
back to top 

Sucampo Pharmaceuticals, Inc. (Sucampo) (Nasdaq:SCMP), a global biopharmaceutical company, and Takeda Pharmaceutical Company Limited (Takeda) today announced that on October 17, 2014, they entered into a global license, development, commercialization and supply agreement for AMITIZA® (lubiprostone). Through this agreement, Takeda expanded its exclusive rights beyond the United States (U.S.) and Canada to further develop and commercialize AMITIZA in all global markets, except Japan and the People's Republic of China.
"Takeda is committed to being a patient and customer-centric organization, making quality health products available to the patients who need them. Through this agreement, AMITIZA can now be made available to patients worldwide," said Shinji Honda, Senior Managing Director and Corporate Strategy Officer. "Takeda forms partnerships to advance science and to provide innovative treatment options for patients, and this global agreement is an excellent example. This global collaboration leverages the expertise we have established through our gastroenterology portfolio of products."
back to top 

Baltimore biopharmaceutical company Profectus BioSciences Inc. has received a three-year, $8.5 million grant from the U.S. Army for work on an Ebola vaccine.
Profectus will share the grant with the Galveston National Laboratory at the University of Texas Medical Branch at Galveston. The grant is Profectus' second in recent days. The company also announced a $5.8 million grant from the Biomedical Advanced Research and Development Authority (BARDA) to conduct safety studies of the company's VesiculoVax, a potential Ebola vaccine.
back to top 

The University of California recently announced its entry into the venture capital arena with a $250 million commitment to spinning promising technologies out of its top-notch, 10-campus system. Perhaps every major research university in the US — collectively, recipients of over $40 billion in federal research funding yearly, not to mention the inflows of corporate research funds — wishes to emulate Stanford’s success in capitalizing on the market successes of university-developed technology (think Google).
back to top 

As a partner at Norwest Venture Partners (NVP), Casper de Clercq has seen countless digital health startups try to get solutions off the ground. He’s also seen many fail.
According to de Clercq, 60 to 70 percent of digital health startups are likely to fail because they are unclear about their go-to market strategy, and don’t have a good understanding of who’s ultimately going to pay for their product.
back to top 

New initiative will support networks that help doctors access information and improve health outcomes
Health and Human Services Secretary Sylvia M. Burwell today announced an initiative that will fund successful applicants who work directly with medical providers to rethink and redesign their practices, moving from systems driven by quantity of care to ones focused on patients’ health outcomes, and coordinated health care systems. These applicants could include group practices, health care systems, medical provider associations and others. This effort will help clinicians develop strategies to share, adapt and further improve the quality of care they provide, while holding down costs. Strategies could include:
back to top 

Funding and Research Opportunities
The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notices:
Notice of Special Accommodations for Submission and Reporting Requirements for Program Directors/Principal Investigators Responding to the West Africa Ebola Outbreak
(NOT-OD-15-010) Office of the Director, NIH
Notice to Extend the Response Date for NOT-OD-14-128 "Request for Information (RFI): Consideration of Sex As a Biological Variable in Biomedical Research"
(NOT-OD-15-012) Office of the Director, NIH
Reminder: NIH Requires the Research Performance Progress Report (RPPR) for All Type 5 Progress Reports
(NOT-OD-15-014) Office of the Director, NIH
Notice of Participation of the National Institute on Drug Abuse in PA-14-334 "Advancing Interventions to Improve Medication Adherence (R01)"
(NOT-DA-14-052)
National Institute on Drug Abuse
NHLBI Announces Frequently Asked Questions (FAQs) for RFA-HL-15-015 "Multi-Site Clinical Trials for the Pulmonary Trials Cooperative (U01)" and RFA-HL-15-016 "Network Management Core (NEMO) for the Pulmonary Trials Cooperative (U01)"
(NOT-HL-14-240)
National Heart, Lung, and Blood Institute
Please note that most links to RFAs, PAs, and Guide Notices will take you to the NIH Web site. RFPs will take you to FedBizOpps. Links to RFPs will not work past their proposal receipt date. Archived versions of RFPs posted on FedBizOpps can be found on the FedBizOpps site using the FedBizOpps search function. Under “Document to Search,” select Archived Documents.
back to top 

Earlier this year, the Secretary of the Department of Health and Human Services (HHS) launched the HHS IDEA Lab. With it, we unveiled a consolidated structure for the innovation activities at the Department of Health and Human Services, flashy new branding and a website. But when we launched, we weren’t totally clear on what the main message for the HHS IDEA Lab was, and over the past 6 months we heard the question – what is the HHS IDEA Lab all about? So we have looked at ourselves, focused on what your needs are to solve problems, become an entrepreneur, or just learn new skills, and have clearly defined what the HHS IDEA Lab is.
back to top 

In early drug discovery, you need a starting point, says Northeastern University associate professor of chemistry and chemical biology Michael Pollastri.
In a new research paper published Thursday in the journal PLOS-Neglected Tropical Diseases, Pollastri and his colleagues present hundreds of such starting points for potentially treating Human African trypanosomiasis, or sleeping sickness, a deadly disease that affects thousands of people annually.
back to top 

The technology for creating new tissues from stem cells has taken a giant leap forward. Two tablespoons of blood are all that is needed to grow a brand new blood vessel in just seven days. This is shown in a new study from Sahlgrenska Acadedmy and Sahlgrenska University Hospital published in EBioMedicine. Just three years ago, a patient at Sahlgrenska University Hospital received a blood vessel transplant grown from her own stem cells.
Suchitra Sumitran-Holgersson, Professor of Transplantation Biology at Sahlgrenska Academy, and Michael Olausson, Surgeon/Medical Director of the Transplant Center and Professor at Sahlgrenska Academy, came up with the idea, planned and carried out the procedure.
back to top 
Regression models, Monte Carlo simulations, and other methods for predicting what’s around the corner have been in use for decades. It’s only recently, though, that advances in information technology have made it possible for predictive tools to access and manipulate big data, and to do so continuously — accelerating the generation of insights, and opening up opportunities to anticipate issues with unprecedented precision. Think of the colleges that are increasingly able to identify students at risk of dropping out and intervene before they do. Or lenders’ enhanced abilities to gauge credit risk. Energy, agriculture, insurance, retail, human resources — no industry is unaffected. But nowhere is the potential of this new era of opportunity more apparent and exciting than it is in health care.
back to top 
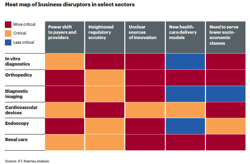
It’s not just the FDA that is making life difficult for medical device companies. Executives are having to follow sales opportunities as medical care shifts out of hospitals into homes and physician offices. They are having to revamp their entire business model to survive in the new world of the ACA.
A.T. Kearney has identified the five forces that are forcing the device industry to evolve in this new report: Medical Devices: Equipped for the Future? In addition to spelling out the threats, the analysts have a guide for how to start building a new business model.
back to top 

Stapling up skin post-surgery is pretty much the norm to quickly seal up wounds, but it runs a risk of infection and injury from the extra damage to already sensitive skin.
Bay Area startup ZipLine Medical has developed a non-invasive but suture-like alternative that it’s positioning as a quicker, simpler and more desirable way to close small surgical wounds. To boot, clinical trials have shown the method decreases both infection likelihood as well as scarring. The company just closed a $5.7 million extension to its Series C financing round, led by a new venture firm in Shanghai called China Materialia that wants to expand the technology there.
back to top 

Massachusetts General Hospital and MIT have formed a $3 million strategic alliance in an attempt to address three “major challenges” that persist in healthcare: improving diagnoses, developing new approaches to prevent and treat infectious diseases and developing more accurate methods of diagnosing and treating neurodegenerative and psychiatric diseases.
The alliance, officials said, will add further heft to already existing efforts between individual collaborations between the two institutions, particularly as they relate to development of diagnostic tools and therapies.
back to top 

Roche plans to spend around $3bn updating and expanding its Basal site, home to the Swiss company's headquarters, over the next 10 years.
The company is set to build a new R&D centre for 1,900 employees and an office building for 1,700 employees, and will also upgrade its existing infrastructure.
back to top 
|