|

Montgomery County, taking a page from the state's playbook, has lured cybersecurity startup Mobile System 7 across state lines from McLean, Va. to Bethesda, Md. with $100,000 of investment and services according to The Washington Post. Expansion of the cybersecurity business sector has become a very high priority throughout Maryland and this is just the latest of a series of investments by the state and local governments to encourage successful cybersecurity companies to open or move businesses to Maryland.
The move is reminiscent of the way Luminal, a cybersecurity startup born in West Virginia, was enticed into moving to Frederick thanks to generous investment offers via the state government. That was state investment however, while the Mobile System 7 move comes after Montgomery County's Department of Economic Development convinced the company that it would do better in Maryland than in Virginia.
back to top 

Supernus Pharmaceuticals, Inc. today announced that United Therapeutics Corporation UTHR +0.58% has paid Supernus a $2 million milestone payment under United Therapeutics' license agreement with Supernus. This payment was due upon the launch of Orenitram™ (treprostinil) Extended-Release Tablets for the treatment of pulmonary arterial hypertension, in the United States. Orenitram™ utilizes a Supernus patented technology platform. In addition to the launch milestone, Supernus will receive royalties on net sales of Orenitram™, and may become entitled to additional milestone payments.
"We are pleased to have played a role in helping to bring Orenitram™ to patients and their physicians as an important new therapy option," said Jack A. Khattar, President and CEO of Supernus. "Over the life of the product, we expect to receive significant recurring royalty revenue from United Therapeutics' commercialization of Orenitram™."
back to top 

Also this week in Pfizer-related Maryland biotech news: Gaithersburg-based GlycoMimetics Inc. collected a $15 million payment from the pharma giant stemming from its license agreement for the sickle cell anemia drug rivipansel.
GlycoMimetics in 2011 inked the Pfizer partnership, worth up to $340 million, to develop rivipansel (then just called GMI-1070) as a treatment for a complication of sickle cell disease called vaso-occlusive crisis.
back to top 

AstraZeneca on Monday rejected a "final" $119 billion buyout offer from Pfizer, possibly sinking a pharmaceutical mega-merger that could have jeopardized jobs at AZ's Maryland subsidiary, MedImmune.
Pfizer, which would have created the world's largest drug company through the deal, on Sunday evening increased its earlier $106 billion cash-and-stock bid. In a statement, AstraZeneca Chairman Leif Johansson called that new offer "inadequate" and said it would have "serious consequences for the company, our employees and the life-sciences sector in the U.K., Sweden and the U.S."
back to top 

The second annual InvestMaryland Challenge, an early-stage business competition of the Maryland Department of Business & Economic Development, came to an exciting close Monday evening at the National Aquarium in Baltimore.
Maryland Governor Martin O’Malley, joined by DBED Secretary Dominick Murray, entrepreneurs and business leaders, applauded the winners of nearly $1 million in grants and prizes.
back to top 

A health IT startup launched last year by four recent Johns Hopkins graduates and one soon-to-be JHU graduate was one of four firms to win a $100,000 top prize Monday in the InvestMaryland Challenge, a state-run competition to help promising new companies in the life sciences and high tech industries.
The competition, run by the Maryland Department of Business and Economic Development, had 260 entries. Healthify took first place in the General Industry category, open to companies anywhere in the U.S. if they open a location in Maryland. Healthify, based in New York, beat out two other finalists—a wholesale food distribution website with a focus on local, sustainably-produced items; and an ad technology company.
back to top 

Engineering is a field with the power to transform lives across the globe, often in the most underserved regions. Nowhere is this maxim more apparent than at the Center for Bioengineering Innovation & Design (CBID), founded in 2007 at the Johns Hopkins University’s Department of Biomedical Engineering.
Each summer, teams of new engineering graduate students travel overseas to areas where poverty and poor healthcare dominate. They look for opportunities to put their skills to work solving difficult, and often deadly, health problems.
back to top 

QIAGEN N.V. (NASDAQ: QGEN; Frankfurt Prime Standard: QIA) today announced the expansion of its industry-leading portfolio of bioinformatics solutions with additional content from BIOBASE, a provider of expertly curated biological databases, software and services. With access to HGMD, an especially in clinical markets widely used biomedical data resource as well as to other unique content, QIAGEN expand its world’s most comprehensive, high-quality and up-to-date literature source for clinical research and diagnosis – further strengthening its market-leading position in the analysis and interpretation of sequencing data. QIAGEN’s growing bioinformatics and next-generation sequencing (NGS) franchises is positioned to benefit from the integration of BIOBASE, its assets and employees and will benefit the expansion of relationships with thousands of clinical labs and NGS users in life sciences.
“The ability of next-generation sequencing to rapidly deliver genomic insights is opening up new frontiers for clinical research and medicine, and QIAGEN is strategically addressing customers’ needs to interpret the massive amounts of data generated by NGS. With HGMD and other content from BIOBASE, a respected organization with a dedicated team and robust line of unique databases and software, QIAGEN is further extending its competitive advantage as the overall market leader for clinical interpretation of human sequencing data,” said Peer M. Schatz, Chief Executive Officer of QIAGEN. “Already today, more than 15,000 users worldwide rely on QIAGEN’s bioinformatics products for interpretation – and have processed over a quarter of a million genome sequences. HGMD is a unique fit with our offering and will integrate well. We expect to drive additional adoption of this leading literature-based knowledge base used by clinical reference labs for annotating hereditary variants, as well as BIOBASE’ other solutions by having integrated them into interpretation solutions shared with our Ingenuity Knowledge Base – adding value for QIAGEN and BIOBASE customers and accelerating our growth drivers in NGS and bioinformatics.”
back to top 

Biotech is certainly a growing industry in Greater Baltimore, and here’s more proof. These 10 companies all said they are growing their staff in the coming months, when we asked in our annual industry survey. Here are all the jobs they’re already looking to fill:
back to top 
 Tuesday, May 27, 2014, 08:30am - 02:00pm Tuesday, May 27, 2014, 08:30am - 02:00pm
SBIR/STTR programs promote small business innovation and profitability while simultaneously meeting the government's research and development needs. Every year, small businesses receive millions of dollars in SBIR/STTR funds for research, development and commercialization purposes. This course will provide attendees with an overview of the SBIR/STTR programs; funding sources and eligibility requirements; best practices in SBIR/STTR proposals writing, involvement, and commercialization; and a discussion of how to protect your company's legal interests in either program.
back to top 
 Join members from NHLBI's SMARTT team for a free webinar to hear how three investigators utilized SMARTT services to advance the development of their lead candidates. Join members from NHLBI's SMARTT team for a free webinar to hear how three investigators utilized SMARTT services to advance the development of their lead candidates.
Webinar agenda
- Overview of SMARTT
- Translational Investigator presentations:
- Steven Idell, MD, PhD, Vice President for Research at The University of Texas Health Science Center at Tyler
Development of fibrinolysin single chain urokinase plasminogen activator (scuPA) for pleural effusions
- Athan Kuliopulos, MD, PhD, Professor of Medicine at Tufts University and Tufts Medical Center
Development of PZ-128 for the prevention of arterial thrombosis in acute coronary syndrome and percutaneous coronary intervention
- Barry Coller, MD, Vice President for Medical Affairs and Physician-in-Chief, The Rockefeller University
Development of novel small molecule platelet inhibitors for pre-hospital treatment of myocardial infarction.
- Challenges in Early Translational Science: Industry Perspective
- Q&A with SMARTT
Representatives from NHLBI staff and from SMARTT's regulatory, manufacturing, and pharmacology/toxicology facilities will be available to answer questions.
back to top 

Translating Ideas from Bench to Bedside
June 3rd, 2014, from 6 to 8:30 pm
Krieger School of Arts and Sciences Advanced Academic Programs
Johns Hopkins University Montgomery County Campus, Rockville, MD 9601 Medical Center Drive (A&R Building, Room 106-110)
Connect with physician entrepreneurs and other stakeholders in healthcare innovation as we explore the winning formula for successfully advancing new medical devices and technology to commercialization.
back to top 

City innovation economy classifications and rankings, 2014.
World’s largest city classification and global ranking with 445 benchmark cities classified, and all cities analyst ranked this year. Measuring each cities potential as an innovation economy at the current time, since 2007.
Based on 2thinknow analyst interpretation of 162 city indicators from 2thinknow City Benchmarking Data set.
back to top 

In almost every case, the scientist-entrepreneurs approaching LSN are falling victim to one or more fallacies that are propagated through the industry. LSN is in a dialogue with over 5,000 investors around the world, and the reality is that what many entrepreneurs believe to be sound business logic could be dooming their companies. This article compiles the top 10 fundraising misconceptions so that you can avoid these pitfalls.
back to top 

Providing shared space, services, equipment, and expertise to budget-conscious scientist-entrepreneurs who are looking to prove a hypothesis and launch a company is the mission of life science incubators. And they have helped many start-ups.
However, increasingly, scientist-entrepreneurs are disappointed with what they’re finding. LSN hears a lot of complaints because we are in dialogue with a lot of the firms that populate these incubators. They acknowledge that incubators are less expensive than going it alone. Still, they say the lab space is too expensive, the promise of seeding seasoned players who can augment the founding team often goes unfulfilled, and there’s little to no tactical fundraising support.
back to top 

American entrepreneurship is apparently on the decline, according to a recent article from FiveThirtyEight, but small business owners report consistently high levels of satisfaction with their choices despite the financial difficulties that they have faced.
“Americans started 27 percent fewer businesses in 2011 than they did five years earlier, according to data from the Census Bureau,” writes Ben Casselman on FiveThirtyEight. As a share of all companies, startups have been declining for more than 30 years.”
back to top 

Vinod Khosla, sassy VC and legendary co-founder of Sun Microsystems, has predicted the future of health.
In essence, he has said, our medical lives will become increasingly automated, with ultra-intelligent systems prescribing fine-grain recommendations to nurse us back to health. According to a newly released report, he predicts:
back to top 

The Angel Capital Association (ACA) today launched a campaign to protect startups from a devastating loss of angel capital if the Securities and Exchange Commission (SEC) increases the financial qualifications for accredited investors. The "Protect Angel Funding" initiative is a call to action to the startup ecosystem—from angel investors to economic development organizations and entrepreneurs – to urge regulators to preserve the definition of who qualifies as an accredited angel investor. Protecting this important investment class is critical to preserving the economic fuel it provides to startups and job creation. If changes proposed by "investor protection" organizations are enacted by the SEC, nearly 60 percent of angel investors would be eliminated.
The Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 requires the SEC to review the definition of an accredited investor in 2014 to determine whether it should be modified "for the protection of investors, in the public interest and in light of the economy." Currently, an individual accredited investor is defined as someone with $1 million in net worth excluding the value of a primary residence, or annual income of $200,000. At issue is whether these financial thresholds should be arbitrarily raised based on inflation.
back to top 

In the US, many entrepreneurs see grants as “free money,” since they are not loans and don’t have to be repaid. A grant is not an equity investment, so the entrepreneur doesn’t have to give up a stake in the company either. Typically they can be used to fund product development and commercialization that would otherwise require outside investors.
A good place to start looking is the Small Business Innovation Research (SBIR) program, which is a lifeline for high-tech startups. A more general approach is to check out Grants.gov, which is a searchable directory of more than 1,000 federal grant programs. An advanced search tool is provided to search for a grant by eligibility, by issuing agency, or category.
back to top 

The DoD SBIR 2014.2 solicitation is now available for the submission of proposals.
Participating Department of Defense (DoD) agencies:
- Army
- Navy
- DARPA
- DLA
- DMEA
- Missile Defense Agency (MDA)
IMPORTANT DATES and DEADLINES
May 23, 2014
Solicitation opens and DoD begins accepting proposals
June 11, 2014
SITIS closes to new questions
June 25, 2014 6:00 am EST
Solicitation closes to receipt of proposals at 6:00 AM EST-plan ahead and submit early.
back to top 
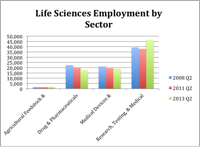
Two year’s ago this month the Fourth Economy team completed an assignment for the Pennsylvania Life Science Leadership Advisory Council. At a news event in May 2012 we participated in the release of “Life Sciences Leadership for the Next Decade: Nurturing a Life Science Ecosystem for Job Creation and Economic Development in Pennsylvania”. This report highlighted five steps and related actions that the life science community could undertake to maintain the economic impact of the life science industry in the state.
Flash forward two years and we wanted to check in and see how the industry was performing in terms of employment and establishments. The following information summarizes our findings. The life science industry continues to be a strong overall sector for the state’s economy.
back to top 

Take a guess as to which industry suffered the most cybersecurity breaches in 2013.
According to one health IT startup, that’s healthcare. Protenus pointed that out at DreamIt Health Baltimore Demo Day in April.
As cofounder Robert Lord explained at Demo Day, electronic medical records, shockingly enough, “weren’t built with security in mind.”
back to top 

Its no secret: the University of Colorado receives hundreds of millions of dollars in federal funds that sponsor our research. My lab alone racks up expenditures of $800,000 a year to pay for post-doc and graduate student salary, tuition, materials and travel. Isn’t it preposterous to now ask the public for some more of their hard-earned cash as we, and many other universities now, do on CU Boulder’s new initiative?
It helps to first think about why the government actually does make these payments in the first place: to educate the next generation of engineers and scientists by working on research problems with important societal benefits. With this money becoming more competitive, the pool of ideas actually being supported becomes smaller with little to no feedback from the general public. Crowdfunding research allows the public to turn this around by enabling scientists to find support for projects currently not (or never) on the radar of conventional funding agencies, yet enjoying sufficient public interest. This category of research is probably the most obviously deserving of crowdfunding and has already enabled a host of documentaries, research excursions, and books, often by providing exclusively ideological or intellectual reward.
back to top 

QIAGEN N.V. (frankfurt prime standard:QIA) today announced that its therascreen® KRAS RGQ PCR Kit (therascreen KRAS test) has received U.S. Food and Drug Administration (FDA) approval to guide the treatment of metastatic colorectal cancer patients with Amgen's Vectibix® (panitumumab). This marks the third FDA approval of a companion diagnostic from QIAGEN that has been paired with a novel medicine.
QIAGEN's growing menu of clinically validated companion diagnostics is driving global dissemination of personalized healthcare, which uses genomic information to guide treatment decisions in individual patients.
back to top 
|