|
 BHI CEO Rich Bendis recently served as an expert panelist contributing to a special report from the "White House Lab-to-Market Inter-Agency Summit." Panelists examined the U.S. federal government's investment in drug R&D and the resulting private sector commercialization. According to the Summit report: BHI CEO Rich Bendis recently served as an expert panelist contributing to a special report from the "White House Lab-to-Market Inter-Agency Summit." Panelists examined the U.S. federal government's investment in drug R&D and the resulting private sector commercialization. According to the Summit report:
Federal research has done exceedingly well at accomplishing its original intent, which is to increase human knowledge, meet mission needs, and undertake high-risk research of long-term importance to the U.S. economy that is beyond the reach of the private sector. But commercialization of resulting discoveries from agency research has largely been an after-thought, despite clear Congressional and Presidential intent expressed through a series of legislative mandates and Executive Orders. While research to meet agency missions is critical, the members of the Panel believe that if the U.S. is to remain globally competitive in the 21st century, it must accelerate the translation of federally-funded R&D into commercial outcomes that create economic and public value, thus maximizing the return on the public dollars invested.
Read more at:
Increasing the Impact of Federally-Funded R&D
back to top 
by Joseph P. Allen and Diane Palmintera
 On May 20, 2013, the White House Lab-To-Market Inter-Agency Summit was held in Washington, D.C. The Summit was organized by the White House Office of Science and Technology Policy and the National Institutes of Health's Heart, Lung, and Blood Institute. The purpose of the meeting was extraordinary: asking national experts outside of the federal agency system to recommend ways to increase the return-on-investment for the $140 billion annual taxpayer expenditure on federally-funded research and development. On May 20, 2013, the White House Lab-To-Market Inter-Agency Summit was held in Washington, D.C. The Summit was organized by the White House Office of Science and Technology Policy and the National Institutes of Health's Heart, Lung, and Blood Institute. The purpose of the meeting was extraordinary: asking national experts outside of the federal agency system to recommend ways to increase the return-on-investment for the $140 billion annual taxpayer expenditure on federally-funded research and development.
The format for the meeting also was unusual. Research agencies nominated 20 national experts experienced in various phases of technology commercialization to participate in the Summit. The Administration placed no preconditions or limitations on the expert Panel and asked it to focus on "transformative" ideas. We were privileged to be asked to serve as the Summit's co-chairs.
The Administration requested that the Panel address several overarching questions:
- How can agencies better align themselves to more effectively promote the commercial development of their research;
- How can effective metrics for various stages of these efforts be developed; and
- How can we better leverage multi-agency resources to enhance the public's return-on-investment through the commercialization of more federally-funded technologies?
back to top 

Topic: "EIRs, SBIRs, and more with BioHealth Innovation, Inc."
Presenters:
- Richard Bendis, President and CEO
- Ethan Byler, Director, Innovation Programs
- Todd Chappell, Entrepreneur-in-Residence, NIH-OTT
- Dr. Ken Malone, Entrepreneur-in-Residence, UMD Ventures
- Ram Aiyar, Entrepreneur-in-Residence, NHLBI
BioHealth Innovation, Inc. (BHI) is a regional innovation intermediary that accelerates and facilitates technology transfer and commercialization of market-relevant research in federal labs, universities, and biohealth companies in the Region. It is a private-public partnership that connects the Region’s innovation assets to provide integrated technical knowledge, financial means, and entrepreneurial/managerial expertise to turn promise into prosperity for the region while advancing human health.
BHI’s Entrepreneur-in-Residence (EIR) program is designed to be an active partner with research institutions to source, fund, and grow high-potential, early-stage products through project-focused companies. The entrepreneurs in the program support the formation of new companies based upon innovative discoveries in the areas of drugs, vaccines, therapeutics, diagnostics, and medical devices from the intramural research programs at the NIH and Food and Drug Administration (FDA), as well as from universities and businesses.
BHI's Commercial Relevance Program (CRP) offers biohealth companies support in preparing applications for federal funding inclusive of SBIRs, STTRs, and other federal government awards. Companies submit their federal funding concepts and receive pre-proposal feedback to help troubleshoot and strengthen your application. Further support from professional consultants and service providers is available to assist in improving your application.
BHI recently published the Central Maryland BioHealth Entrepreneur's Resource and Finance Guide 2013. The Guide serves as a compendium of resources to biohealth innovators and entrepreneurs working to start and grow new companies and technologies in the region.
back to top 

The INNo program trains research scientists in the entrepreneurial skills needed to bring technology inventions and services to the healthcare market.
Participants in the INNo program learn to:
- Identify and evaluate the commercial potential of intellectual property
- Understand the business fundamentals related to technology start-ups
- Create a value proposition and business concept for a new product, platform, or service
- Articulate investment opportunities persuasively to potential investors and partners
- Develop a network of resources in the Maryland entrepreneurial community
back to top 
 The RESI conference is poised to be one of the more unique events in the life sciences space this coming fall. This full-day investor partnering conference is groundbreaking in that it is focused on redefining the investor landscape in early stage life sciences. As all of us in the industry are aware, the life science investor landscape has changed; venture capital has left a void and there is a plethora of new entities entering the space with capital to allocate. The RESI conference is poised to be one of the more unique events in the life sciences space this coming fall. This full-day investor partnering conference is groundbreaking in that it is focused on redefining the investor landscape in early stage life sciences. As all of us in the industry are aware, the life science investor landscape has changed; venture capital has left a void and there is a plethora of new entities entering the space with capital to allocate.
This conference has assembled these players - Senior decision-makers from some of the largest pharmaceutical & device companies, patient groups, philanthropic organizations, investment banks, and family offices will all be joining the action on September 16th. The conference will also have representation from next-generation technology transfer, licensing and funding experts, and there will be a free fund-raising boot camp. We urge all biotech and medtech readers to take a look at the program, and to take some time out to reeducate themselves regarding the new landscape unfolding in the life science investor arena.
Biohealth Innovation has been able to secure Earlybird pricing for our readership through August 30th via this link - We look forward to meeting you in September!
back to top 
 Kauffman Foundation FastTrac®, has joined with Montgomery College to support future and current business owners before, during, and after the startup process. Entrepreneurs will receive the information, resources, and networks necessary to start and grow successful businesses. Kauffman Foundation FastTrac®, has joined with Montgomery College to support future and current business owners before, during, and after the startup process. Entrepreneurs will receive the information, resources, and networks necessary to start and grow successful businesses.
Three courses will be offered:
- FastTrac NewVenture
- FastTrac GrowthVenture
- FastTrac TechVenture
For more information: Program Flyer
back to top 

University of Maryland (UM) Ventures announced today agreements between University of Maryland, Baltimore (UMB) and five different life sciences companies across the Baltimore/Washington metropolitan region. The companies include Montgomery County-based Rexahn Pharmaceuticals, Baltimore County-based Plasmonix, Prince Georges County-based IGI Technologies, Howard County-based A&G Pharmaceuticals, and Frederick County-based BioAssay Works. These deals are part of UM Ventures' continual efforts to accelerate technology commercialization, advance industry collaboration, and support projects with commercial value at both the Baltimore and College Park campuses of the university.
"UMB is very excited to collaborate with these companies, each an innovator in its own right," said Phil Robilotto, Assistant Vice President, Office of Technology Transfer, UMB. "These types of collaborations are at the core of our mission to channel the expertise of our industry partners and highlight our efforts to support the Maryland biotechnology community."
back to top 

The following funding opportunity announcements from the NHLBI or other components of the National Institutes of Health, might be of interest:
NIH Guide Notice:
NOT-OD-13-095): Using ASSIST to Prepare and Submit Multi-Project Applications to NIH: Webinar - August 13, 2013
NIH will require electronic submission for all P01, P20, P50 and U19 applications intended for due dates on or after September 25, 2013.
NOT-OD-13-097: Extension of eRA Commons User IDs to Individuals in Graduate and Undergraduate Student Project Roles with Measurable Effort on an NIH Annual Progress Report (PHS2590 & RPPR)
Over the next year the NIH will start requiring an eRA Commons ID for all individuals in graduate and undergraduate student roles who participate in NIH-funded projects for at least one person month or more.
NOT-HL-13-187: Notice of Intent to Publish a Funding Opportunity Announcement for Low-Cost Pragmatic Patient-Centered Randomized Controlled Intervention Trials (UH2/UH3)
The NHLBI, along with other NIH Institutes and Centers, intends to promote a new initiative by publishing a Funding Opportunity Announcement (FOA) to solicit applications to plan and conduct low cost, pragmatic, randomized clinical trials that are integrated into existing clinical practice settings and/or leverage existing electronic patient care resources.
Program Announcement (PA):
PA-13-302: Research Project Grant (Parent R01) (expires September 8, 2016)
The Research Project Grant (R01) supports a discrete, specified, circumscribed project to be performed by the named investigator(s) in areas representing the specific interests and competencies of the investigator(s). The proposed project must be related to the programmatic interests of one or more of the participating NIH Institutes and Centers (ICs) based on descriptions of their programs.
PA-13-292: Behavioral and Social Science Research on Understanding and Reducing Health Disparities (R01) (expires September 8, 2016)
The purpose of this FOA is to encourage behavioral and social science research on the causes and solutions to health and disabilities disparities in the U. S. population.
back to top 

About one year ago GlaxoSmithKline (NYSE: GSK) set up a division to study the electrical impulses along the peripheral nervous system and develop technologies to read, change, or manipulate these impulses to treat acute and chronic diseases. Now it’s launched a $50 million venture capital fund to invest in companies with pioneering technology in this emerging field of bioelectronics, according to a company statement.
The Action Potential Venture Capital fund, which will be based in Cambridge, Massachusetts, takes its name from the electrical signals that pass along the nerves in the body. Problems with the patterns of these impulses are associated with a broad range of diseases.
back to top 

When PharmAthene Inc. merges with Seattle-based Theraclone Sciences Inc., it will become part of a company that has the kind of deal with Big Pharma any drug discovery firm would envy.
Theraclone has a multimillion dollar research and development deal with pharmaceutical giant Pfizer Inc. that could be worth as much as $632 million in license, royalty and other payments to the combined company. Theraclone and PharmAthene are entering into an all-stock merger of equals. The combined company will trade publicly.
back to top 

Leggett Joins O’Malley/Brown Administration in Announcing $630 Million Investment in County’s Transportation Network; Governor Also Announces Additional $400 Million for Construction of Purple Line for Montgomery and Prince George’s
Montgomery County Executive Ike Leggett today joined Governor Martin O’Malley and Lt. Governor Anthony Brown during their announcement that Montgomery County will be receiving $628 million in transportation investments and an additional $400 million for construction of the Purple Line that will benefit both Montgomery and Prince George’s counties. Brown also announced that the Purple Line will be built as a public-private partnership under HB 560, the law he championed to attract private investment for new infrastructure in Maryland.
back to top 

The Johns Hopkins University’s schools of medicine and public health received a $5.8 million federal government grant for research on frailty among older adults. The five-year grant will allow the university to continue research designed to identify the causes of frailty in older adults and speed the development of interventions to slow or stop it.
The grant renews funding of the Johns Hopkins Claude D. Pepper Older Americans Independence Center (OAIC ), a federally designated center of excellence that is one of only 14 such university sites nationwide supported by the National Institute on Aging (NIA ). The centers are named for a longtime Democratic member of Congress who championed support for older adults.
back to top 

The University of Maryland announced Monday that long-time finance professor Alexander J. Triantis has been appointed dean of the Robert H. Smith School of Business.
Triantis, 49, succeeds G. "Anand" Anandalingam, who left the post at the end of June to take a position in London as dean of the Imperial College Business School. Triantis will assume the position Sept. 1.
back to top 

Governor Martin O'Malley called attention to state investments in technology during a visit on July 31 to the University of Maryland BioPark, where he spent time in corporate laboratories with chief executive officers and their employees.
Jay A. Perman, MD, president of the University of Maryland, Baltimore (UMB), joined O'Malley on the tour and at a news conference, where the governor spoke about the importance of supporting the life sciences. O'Malley noted that the state has a plan to invest $1.3 billion in life sciences by 2020 and has increased tax incentives to encourage biotechnology and research and development.
back to top 

The University of Maryland University College announced Wednesday it will be the first in the state’s university system to create a path for students to earn academic credit for learning through “massive open online courses.”
The university is one of the nation’s largest public providers of online higher education with an enrollment of about 93,000 students.
back to top 

Digital health accelerator Healthbox and anchor partner Florida Blue had their priorities straight when they chose the seven companies for their inaugural Florida accelerator program: Address the high populations of seniors and youths in the state.
Companies that do that will have more opportunity to scale and build momentum, they reasoned.
back to top 

No factor defines success and failure for a drug company more than this: Companies that invent more, better drugs at a lower cost do better than those that hemorrhage cash but never get an important product to market. Yet 19 in 20 medicines in experimental development fail, meaning a great many companies fail too.
For years, researchers, including one team from Tufts University and another at Eli Lilly, have estimated the cost of inventing and developing a drug at $1 billion or more. These estimates try to exclude costs not directly related to a drug’s approval and also don’t allow for any comparisons between companies. Last year, for the first time, I did something far cruder: I took the 15-year research spending of a group of big pharmaceutical companies and divided it by the number of new drugs (technically new molecular entities, the Food and Drug Administration’s term for drug molecules that have not been approved in any form for any use previously).
back to top 

The Office of Translational Alliances and Coordination (OTAC) in the Division of Extramural Research Activities (DERA), NHLBI is seeking outstanding candidates for the Business Development Specialist (Health Scientist Administrator) position. The OTAC is charged with accelerating the translation of basic discoveries and innovations into new diagnostics, devices, and therapeutics, and facilitating the development of new technologies via Small Business Innovation Research (SBIR) initiatives. The Office facilitates identification of emerging areas of translational opportunities and provides functional integration by developing interdependent teams that leverage resources and intellect across the NHLBI, and with other NIH Institutes, agencies, and organizations. The OTAC enhances communication and coordination between existing programs, develops and coordinates strategic initiatives and Funding Opportunity Announcements (FOAs), and identifies and capitalizes on synergies to meet and enhance program goals. For more information about the OTAC, please visit http://www.nhlbi.nih.gov/about/dera/otac.
The Business Development Specialist will be responsible for the evaluation of the scientific and technological novelty, business opportunity potential and commercialization merits for research projects in the NHLBI SBIR/STTR portfolio as well as other initiatives in the OTAC. Responsibilities of the position include activities such as portfolio management assistance; drafting solicitations; evaluating the effectiveness of short and long-term SBIR projects, providing advice to the OTAC Director and NHLBI senior-level scientists regarding strategic SBIR technology development; establishing internal and external contacts to foster the development of programs and identification of opportunities for SBIR technology research support and collaboration; facilitating scientific collaborations between NHLBI, NIH, DHHS and other Federal agencies, industry and the private sector; and building public-private partnerships to ensure best practices and exchange information.
back to top 

Kleiner Perkins, the investor behind Google and Amazon, and Accel Partners, best known for its investment in Facebook, are putting $18 million into MyFitnessPal, a little-known digital-health startup that has helped its 40 million-plus users shed a collective 100 million pounds.
Despite those accomplishments, this is the first time MyFitnessPal has raised money from professional investors, which raises the question: Do venture capitalists have any idea what they're doing in the networked fitness market?
back to top 

You may have a great idea for a new diagnostic, drug, or technology but developing, testing, and commercializing it takes money. One very viable source for a biotech start-ups in the US are SBIR and STTR Small Business Technology Development grants.
These federal grant programs provide essential funds for hundreds of entrepreneurs to get their technology out of the lab into the market. In fact, with awards totaling about $700 million per year, the SBIR/STTR program is one of the leading sources of seed funds for start-ups and small businesses developing new disease and health related technologies.
back to top 

Some hospitals are turning to technology entrepreneurs to reduce readmission rates and avoid penalties imposed under the Affordable Care Act, the Wall Street Journal reports.
Background
Last year, an ACA provision went into effect that allows CMS to penalize hospitals for excess readmissions of Medicare patients.
The penalties are based on the number of heart failure, heart attack or pneumonia patients above the national average who are readmitted within 30 days to an acute-care hospital.
back to top 

For most of us, the blister packs our medicines come in are just temporary barriers to be scratched open with our fingernails or popped open like Chiclets. We usually don’t even pay attention to the tiny, vaguely printed expiration dates tattooed on the silver skin of our aspirin or cough medicine’s packaging; we take for granted that it’s in date.
Yet around the world, billions of people can’t take the expiration dates of their medication for granted. Doing so can, and often is, fatal. A new concept could put an end to that by encapsulating our medicines in strips that change color as they expire, transforming the packaging of dangerously out-of-date medication into a chromatic warning. But will big pharma bring it to market?
back to top 

Money may not make the world go round, but it does keep companies innovating. The Small Business & Technology Development Center has made formal a longstanding relationship with BBCetc., an Ann Arbor-based development consulting company with a specialty in helping businesses secure federal grant funding for commercialization and growth.
The partnership will utilize BBCetc.’s extensive background in federal research grant proposals with the SBTDC’s statewide organization and presence to assist companies with writing proposals specifically for the Small Business Innovation Research and Small Business Technology Transfer federal research grants.
back to top 

Proof of Concept grants are intended to enhance the commercial viability of health-related technologies or concepts developed by non-profit organizations or enhance the competitiveness of early-stage companies for private equity investment. The maximum award is $250,000 in total costs.
Submission and review process:
- Three cycles of pre-proposal review, proposal review, and awards by the LSDF Board of Trustees through August 2014. Up to 32 pre-proposals will be reviewed per cycle.
- Pre-proposals are reviewed every four months and require a presentation and interview.
- Invited proposals are due one month after pre-proposal reviews and reviewed the following month. Proposal reviews require a presentation and interview.
- Awards are made one month after proposal reviews.
- If a pre-proposal results in an invitation to submit a proposal, that invitation is valid only for the proposal deadline immediately following the pre-proposal review.
back to top 

The University of Maryland College Park ranks among the best universities in the nation, but it appears the Terps are known to let their hair down.
College Park also ranks among the top 20 party schools in the country, according to the newly released rankings from the Princeton Review.
back to top 
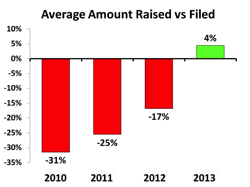
It isn’t just the volume of IPOs this summer that makes this window a victory for hibernating private biotechs and their VC backers, it’s the details of the offerings in terms of price, performance, and type of biotech making the leap onto the public stage. For the last five years we have not seen average offering prices land above expectations, but 2013 is breaking that trend. Price performance is also impressive, with a number of IPOs hovering near 2x the offering price. Rounding out these two positives for valuation is the return of the formerly taboo Phase I and Pre-Clinical IPOs – a qualitative signal of strength.
back to top 

GlaxoSmithKline's new $50 million venture capital fund will be based out of the One Broadway building in Cambridge's Kendall Square, inside an office of SR One, the drug giant's corporate VC arm, a GSK spokesperson said.
The 16-floor One Broadway building also houses the Cambridge Innovation Center startup offices across a number of its floors, as well as venture capital firms Charles River Ventures and Highland Capital Partners.
back to top 

MedCity News writes about all kinds of startups whether their pitch is more at home on CNN or MIT’s Technology Review. We are always so focused on what’s coming next that we forget to reflect on all the companies we have recently discovered. To solve this, we’ve created a monthly report to make sure none of our readers missed any of these posts.
Our new Monthly Startups Index is a free (e-mail registration required) compendium of the early-stage activity across healthcare. It is a piece of business intelligence that includes MedCity’s deeper looks at select early-stage companies, chronicles the investment activity and other news, and even highlights which startups got the most attention from MedCity readers every month.
back to top 

Patients like it and so do health organizations, but electronic communications in clinical care will likely not be widely adopted by primary care physicians unless patient workloads are reduced or they are paid for the time they spend phoning and emailing patients, both during and after office hours.
Those are some key conclusions of an in-depth examination by investigators at Weill Cornell Medical College of six diverse medical practices that routinely use electronic communication for clinical purposes. The detailed report, the most comprehensive of its kind, appears in the August issue of the journal Health Affairs.
back to top 

A person who has suffered from a stroke or spinal-cord injury might need to use crutches or a wheelchair as they gradually regain lost motion through physical therapy.
But these patients could see drastically different effects strapping on a robotic bodysuit or a bionic limb and walking around like Iron Man as they heal. And such digital hardware has the potential to make them recover faster, as well.
back to top 
|