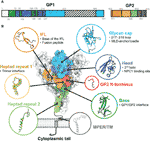

Monoclonal antibodies (mAbs) have proven effective for the treatment of ebolavirus infection in humans, with two mAb-based drugs Inmazeb™ and Ebanga™ receiving FDA approval in 2020. While these drugs represent a major advance in the field of filoviral therapeutics, they are composed of antibodies with single-species specificity for Zaire ebolavirus. The Ebolavirus genus includes five additional species, two of which, Bundibugyo ebolavirus and Sudan ebolavirus, have caused severe disease and significant outbreaks in the past. There are several recently identified broadly neutralizing ebolavirus antibodies, including some in the clinical development pipeline, that have demonstrated broad protection in preclinical studies.
Image: https://www.frontiersin.org