|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
<!doctype html> BioHealth Innovation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|


We hope you and your families are well. This is a reminder that due to COVID19 the BioHealth Capital Region Forum, originally scheduled to take place this week, April 14th and 15th at AstraZeneca, has been postponed.
We are looking into combining the BioHealth Capital Region Forum with the BioHealth Capital Region Investment Conference which tentatively will be held between October 19th and 21st, 2020. We will advise you of our future direction on these events as soon as we develop our path forward. Please save these dates until we finalize the details as we hope to have an in-person event at that time. Thank you for your patience and understanding during these challenging times.
– BioHealth Capital Region Forum Planning Committee

Rockville, Md. — In a greater effort to ensure that the Maryland National Capital Region can emerge as quickly as possible from the expected economic hardships of the COVID-19 coronavirus, the Montgomery County Economic Development Corporation (MCEDC) announced today that it has joined with its state peers in the Greater Washington metropolitan area to form a regional alliance to support an economic recovery.
A Joint Statement of collaboration formalizing the Maryland National Capital Region Economic Development Alliance (MNCREDA) was signed virtually by the leaders of the economic development organizations representing six counties. The Joint Statement document and a photo of its virtual signing are attached.
Image: https://thinkmoco.com


BALTIMORE, MD (April 13, 2020) – Aphena Pharma Solutions, an industry leading organization that provides packaging and manufacturing solutions for the medical industry, is planning to expand its pharmaceutical manufacturing operations in the Town of Easton. The company, which currently operates within a 110,000 square foot facility at 7978 Industrial Park Road, is purchasing an additional 54,000 square foot facility adjacent to its existing location. Building renovations and the installation of new equipment is expected to begin this year, with an estimated completion time of December 2020. Aphena currently employs 150 full-time workers and plans to add an additional 100 jobs in Talbot County over the next two years.
![]()
In response to the COVID-19 crisis, emocha Mobile Health has implemented a remote monitoring service powered by human engagement to support health systems including Johns Hopkins Medicine and LifeBridge Health-affiliated hospitals. emocha uses short, asynchronous video check-ins to identify, track, and manage symptoms of healthcare professionals who have been exposed to COVID-19.
Healthcare workers are on the front lines of the COVID-19 (coronavirus) pandemic, and ensuring their safety and availability is critical. The virus is spreading rapidly across the globe with more than 35,350 confirmed cases in the United States as of March 23. As a result, this highly infectious and novel disease requires strategies to protect the public’s health and to slow the rate of transmission.
![]()
BALTIMORE, MD March 24, 2020 – PathSensors Inc, a Baltimore biotechnology company, announced today that they are developing a CANARY ™ biosensor to detect the Novel SARS Coronavirus (SARS-CoV-2), the causal agent for the coronavirus disease COVID–19. The biosensor will be available for research purposes in May 2020 and validation data on the new SARS-CoV-2 product will be available June 2020.
CANARY ™ technology provides a unique opportunity to reduce the impending public health impact of COVID-19 with rapid results and high specificity. The initial application for this PathSensors product will be for testing of environmental swabs and air monitoring in sensitive spaces such as hospitals, offices, food services, etc. PathSensors will continue to advance the assay’s capabilities as the novel virus is further characterized. As the SARS-CoV-2 biosensor is commercialized, PathSensors expects new uses to emerge, such as rapid specimen screening.


As an entrepreneur mentor and startup investor, I see with sadness the 50 to 90 percent that fail. If you ask them for a reason, most will insist that they couldn’t get funding, or they ran out of money too early. But I’m not convinced that it’s as simple as that. Many are just not facing the reality that their passion had a critical business flaw.
Image: https://blog.startupprofessionals.com


Everybody thinks they’re a leader, but most are far from it. Successful startups, at all levels, depend on leadership and the ability of a few to organize both day-to-day and long-term goals. On the face of it, this sounds simple, but the reality is that not everyone is cut out to lead and to run a successful startup. It requires a certain mindset and armory of skills. These eight tips can help you lead your team.
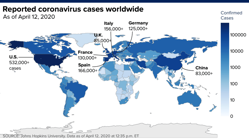

Nobel-prize winning economist Robert Shiller warns a pandemic of fear could tip the economy into an undeserved depression.
Shiller, an expert in how our emotions drive financial decisions, finds the sheer volume of chatter surrounding depression risks due to the coronavirus could severely hurt the economy.
Image: https://www.cnbc.com