

The Trade and Cooperation Agreement and other agreements below are provided for information only. No rights may be derived from them until the date of application. The numbering of the articles is provisional.


The Trade and Cooperation Agreement and other agreements below are provided for information only. No rights may be derived from them until the date of application. The numbering of the articles is provisional.

GA ITHERSBURG, MD — January 04, 2021 – NexImmune, a clinical-stage biotechnology company developing a novel approach to immunotherapy designed to employ the body’s own T cells to generate a specific, potent and durable immune response that mimics natural biology, today announced the hiring of veteran biopharmaceutical industry leader Jerome (Jerry) Zeldis, MD, PhD, as Executive Vice President of Research & Development and the appointment of leading immunotherapy expert Jeffrey Weber, MD, PhD, as Chief Scientific Advisor and Scientific Advisory Board Chair.
ITHERSBURG, MD — January 04, 2021 – NexImmune, a clinical-stage biotechnology company developing a novel approach to immunotherapy designed to employ the body’s own T cells to generate a specific, potent and durable immune response that mimics natural biology, today announced the hiring of veteran biopharmaceutical industry leader Jerome (Jerry) Zeldis, MD, PhD, as Executive Vice President of Research & Development and the appointment of leading immunotherapy expert Jeffrey Weber, MD, PhD, as Chief Scientific Advisor and Scientific Advisory Board Chair.
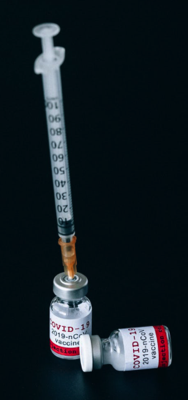

COVID-19 vaccine research drove a lot of funding to biotechnology companies in Maryland’s Montgomery County last year, but it didn’t all come from coronavirus research.
Life sciences companies based in, or with a presence in, Maryland’s largest county received nearly $7.7 billion in research and development funding from the federal government, private investors and nonprofit organizations in 2020, according to the Montgomery County Economic Development Corp., a public/private partnership aimed at helping private companies connect with funding, permits and other resources.


The Johnson & Johnson Nurses Innovate QuickFire Challenge in Mental Health, together with the American Psychiatric Nurses Association (APNA), invites nurses and nursing students worldwide to submit their nurse-led novel concepts, education programs, protocols, prevention or treatment approaches, screening tools, and consumer product ideas with the power to potentially transform mental health care and well-being for their fellow healthcare professionals or the patients they serve amid the current pandemic environment and beyond.
Areas of interest


ROCKVILLE, Md., Jan. 6, 2021 /PRNewswire/ — REGENXBIO Inc. (Nasdaq:RGNX), a leading clinical-stage biotechnology company seeking to improve lives through the curative potential of gene therapy based on its proprietary NAV® Technology Platform, today announced that it intends to offer and sell, subject to market conditions, $175,000,000 of its common stock in an underwritten public offering. The offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed, or the actual size or terms of the offering. In addition, REGENXBIO intends to grant the underwriters a 30-day option to purchase up to an additional 15% of the number of common shares sold in connection with the offering.
BofA Securities, Morgan Stanley and Barclays are acting as joint book-running managers of the offering.


The Johns Hopkins Coronavirus Resource Center, a site launched in the spring of 2020 to offer critical data and perspective during the pandemic, logged its billionth page view today.
Launched March 3, the Coronavirus Resource Center has become a trusted destination for data on the spread and reach of COVID-19. Its continuously updated data trackers and tools help the public, policymakers, and health care professionals worldwide respond to the pandemic. The site includes the latest numbers on cases, testing efforts, and the vaccine rollout as well as expert analysis.


Emergent BioSolutions (EBS) – Get Report shares rose Wednesday after the life sciences company and Mount Sinai Health System announced a trial for Emergent’s coronavirus preventative with $34.6 million of support from the Pentagon.
Emergent recently traded at $92.70, up 2.84%, and has surged 71% year to date amid investor enthusiasm for companies involved with Covid treatments.


Washington, D.C.-based Children’s National Hospital is partnering with the National Institutes of Health and tech firm NVIDIA to launch a challenge in which participants will develop artificial intelligence tools to improve the detection of COVID-19 in the lungs, the hospital announced Jan. 7.
Participants in the COVID-19 Lung CT Lesion Segmentation Grand Challenge will use a multi-institutional, multinational NIH dataset that houses data on patients with a diverse range of age, gender and disease severity to create AI-powered imaging models to help clinicians better identify and treat COVID-19 infection in patients’ lungs.


Richmond, VA, Jan. 07, 2021 (GLOBE NEWSWIRE) — The Center for Innovative Technology (CIT) announced today its Request for Proposals (RFP) for the first solicitation under the newly formed Commonwealth Commercialization Fund (CCF).
The CCF was launched on July 1, 2020 to foster innovative and collaborative commercialization efforts in Virginia, consolidating two legacy programs: the Commonwealth Research Commercialization Fund (CRCF) and the Virginia Research Investment Fund (VRIF). In FY2021, CIT is conducting a single CCF solicitation for young Virginia companies that have strong potential to drive economic growth in Virginia, including through revenue and job creation. Up to $7 million is available to award; a one-to-one match is required.
This competitive round will provide grants up to $100,000 to support companies’ commercialization and market entry goals through product or service development, market research, intellectual property protection, marketing, pilots, and more. High-potential projects are sought in seven strategically important sectors: agricultural and environmental technologies, autonomous systems, clean energy, cybersecurity, data science and analytics, life and health sciences, and space and satellites. All Virginia companies that meet the eligibility criteria are invited to seek funding.


US healthcare venture fundraising soared in 2020, totaling $17B, a 57% increase over 2019’s record.
Image: https://www.svb.com